KBay Dec 4 Physiology
Physiology Processing for Dec and July 2019 time points
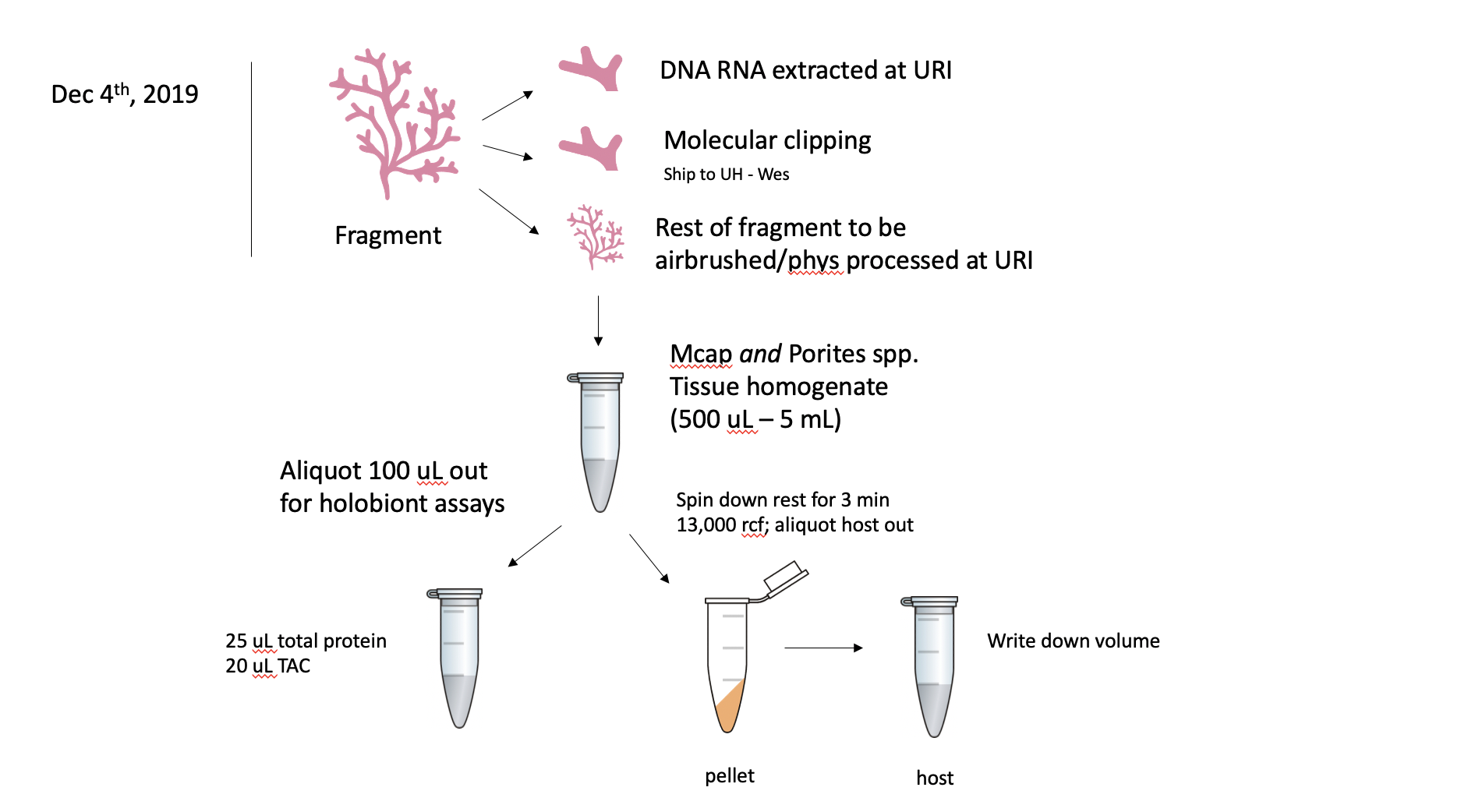
KBay Bleaching Pairs Timeseries Project. Dec and July 2019: after clipping and extractions, the rest of the fragments will be airbrushed and processed for physiology metrics at URI. This is only for M. capitata. P. compressa clippings to stay at URI for future molecular analyses and fragment will not be airbrushed at this time. All fragments were clipped and clipping sent to UH for microbial analyses.
Molecular processing: DNA RNA extractions notebook post here.
Physiology excel spreadsheet with physiology processing information here.
Contents:
Master List with airbrushing processing
| ColonyID | Species | Site | Pair | Bleach | Date | Airbrush Date | volume (mL) | Notes |
|---|---|---|---|---|---|---|---|---|
| 3 | Montipora capitata | 13 | 1 | Bleach | 7/16/19 | |||
| 4 | Montipora capitata | 13 | 1 | Non-bleach | 7/16/19 | |||
| 11 | Montipora capitata | 13 | 2 | Bleach | 7/16/19 | tiny | ||
| 12 | Montipora capitata | 13 | 2 | Non-bleach | 7/16/19 | 20220411 | 8.5 | |
| 19 | Montipora capitata | 13 | 3 | Bleach | 7/16/19 | 20220411 | 8 | |
| 20 | Montipora capitata | 13 | 3 | Non-bleach | 7/16/19 | tiny | ||
| 201 | Montipora capitata | 13 | 9 | Bleach | 7/16/19 | NA | NA | no more fragment |
| 202 | Montipora capitata | 13 | 9 | Non-bleach | 7/16/19 | |||
| 203 | Montipora capitata | 13 | 10 | Bleach | 7/16/19 | 20220411 | 2 | tiny; no extra homogenate; double the SA for 2 fragments b/c half of skeleton crumbled in bag |
| 204 | Montipora capitata | 13 | 10 | Non-bleach | 7/16/19 | |||
| 209 | Montipora capitata | 13 | 11 | Bleach | 7/16/19 | 20220411 | 3 | |
| 210 | Montipora capitata | 13 | 11 | Non-bleach | 7/16/19 | 20220411 | 7.5 | |
| 211 | Montipora capitata | 13 | 12 | Bleach | 7/16/19 | 20220411 | 4.5 | |
| 212 | Montipora capitata | 13 | 12 | Non-bleach | 7/16/19 | |||
| 217 | Montipora capitata | 13 | 13 | Bleach | 7/16/19 | |||
| 218 | Montipora capitata | 13 | 13 | Non-bleach | 7/16/19 | 20220411 | 7 | |
| 219 | Montipora capitata | 13 | 14 | Bleach | 7/16/19 | 20220411 | 7 | |
| 220 | Montipora capitata | 13 | 14 | Non-bleach | 7/16/19 | 20220411 | 12.5 | 11 + 1-2 mL leaked out |
| 221 | Montipora capitata | 13 | 15 | Bleach | 7/16/19 | 20220411 | 11.5 | |
| 222 | Montipora capitata | 13 | 15 | Non-bleach | 7/16/19 | |||
| 3 | Montipora capitata | 13 | 1 | Bleach | 12/4/19 | 20220317 | 9.5 | |
| 4 | Montipora capitata | 13 | 1 | Non-bleach | 12/4/19 | 20220321 | 24 | 2 tubes |
| 11 | Montipora capitata | 13 | 2 | Bleach | 12/4/19 | 20220321 | 13.5 | 10 + 3-4 mL spilled out |
| 12 | Montipora capitata | 13 | 2 | Non-bleach | 12/4/19 | 20220411 | 9.5 | fragment crumbled |
| 19 | Montipora capitata | 13 | 3 | Bleach | 12/4/19 | 20220321 | 26.5 | 2 tubes |
| 20 | Montipora capitata | 13 | 3 | Non-bleach | 12/4/19 | 20220321 | 28 | 2 tubes |
| 201 | Montipora capitata | 13 | 9 | Bleach | 12/4/19 | 20220321 | 13 | |
| 202 | Montipora capitata | 13 | 9 | Non-bleach | 12/4/19 | 20220321 | 11 | |
| 203 | Montipora capitata | 13 | 10 | Bleach | 12/4/19 | 20220411 | 14 | |
| 204 | Montipora capitata | 13 | 10 | Non-bleach | 12/4/19 | 20220321 | 17.5 | 2 tubes |
| 209 | Montipora capitata | 13 | 11 | Bleach | 12/4/19 | 20220321 | 11.5 | 7 + Bag leaked ~4-5 mL spilled out |
| 210 | Montipora capitata | 13 | 11 | Non-bleach | 12/4/19 | 20220321 | 4.5 | |
| 211 | Montipora capitata | 13 | 12 | Bleach | 12/4/19 | 20220321 | 12 | |
| 212 | Montipora capitata | 13 | 12 | Non-bleach | 12/4/19 | 20220321 | 8 | |
| 217 | Montipora capitata | 13 | 13 | Bleach | 12/4/19 | |||
| 218 | Montipora capitata | 13 | 13 | Non-bleach | 12/4/19 | 20220411 | 16 | 2 tubes |
| 219 | Montipora capitata | 13 | 14 | Bleach | 12/4/19 | 20220321 | 15 | |
| 220 | Montipora capitata | 13 | 14 | Non-bleach | 12/4/19 | 20220321 | 7 | |
| 221 | Montipora capitata | 13 | 15 | Bleach | 12/4/19 | 20220321 | 10 | |
| 222 | Montipora capitata | 13 | 15 | Non-bleach | 12/4/19 | 20220321 | 10.5 |
Two of the skeletons don’t really look like M. capitata skeletons.. I don’t have any notes about these not looking right during extractions but circle back to this to double check these are actually Mcap..?
Cell counts
Chlorophyll concentration
Example Processing Workflow - not used as the final version for this project
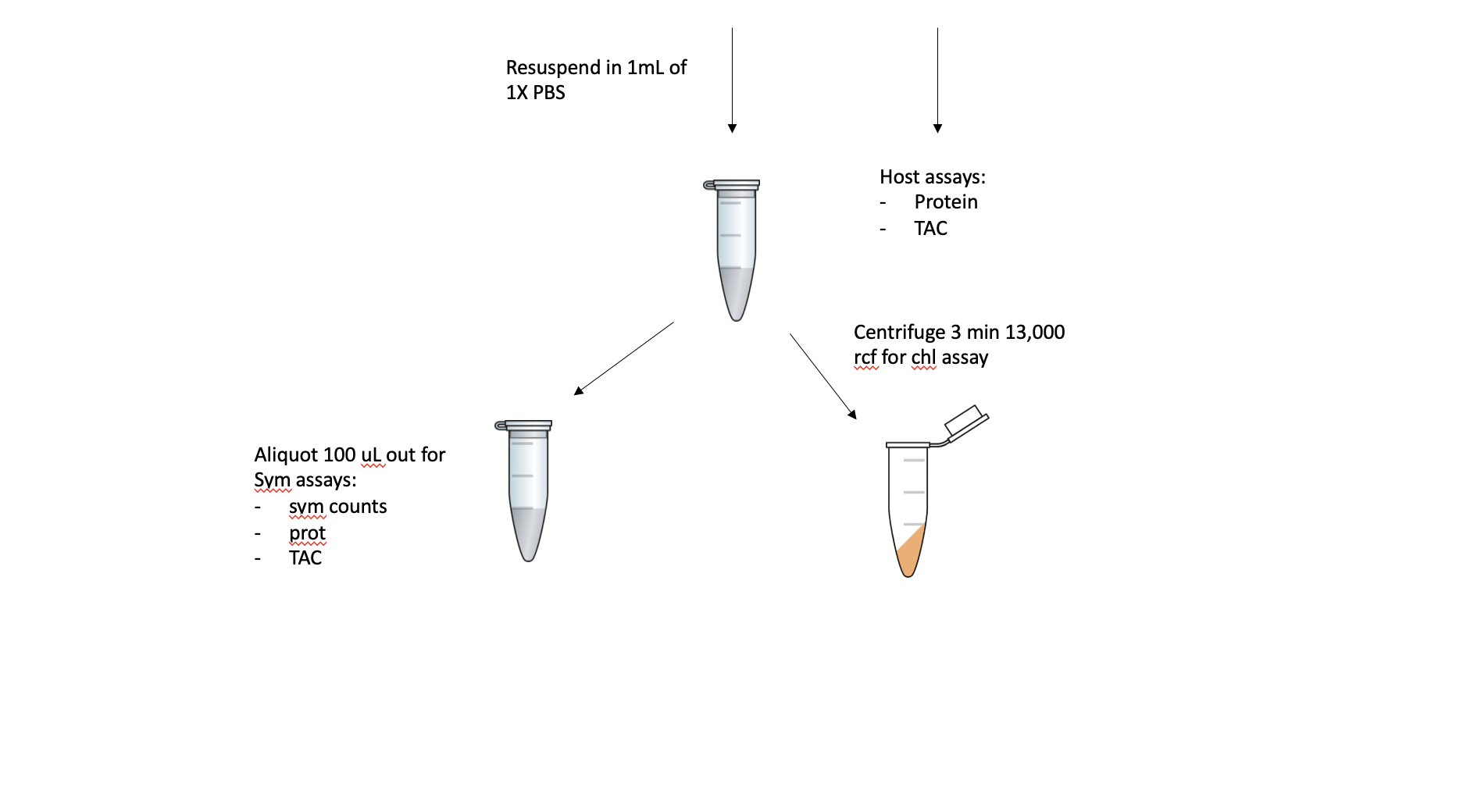
After the URI and UH clipping, the fragment will be airbrushed with 1X PBS. The tissue slurry will be homogenized and 100 uL will be aliquoted out for holobiont assays (i.e. total protein, TAC) before being centrifuged for 3 min at 13,000 rcf. The host fraction will be aliquoted out and volume recorded, and this can be used for host assays (i.e. protein, TAC). The pellet will be resuspended in 1 mL of 1X PBS. From this aliquot, 100 uL will be separated for Sym assays (symbiont counts, protein, TAC) and the remaining 900 uL will be used in a chlorophyll content assay.
The holobiont, host, and symbiont fractions saved can be used for future assays we want to do like lipid content.
If the volume is >2 mL for a coral fragment, then only 1 mL will be spun down following the 100 uL holobiont assay aliquot.
Alternative protein kit:
https://benchling.com/s/prt-kaJmsboePaIXC1R9aATC/edit
