Holobiont Integration July DNA RNA Extractions
July DNA RNA Extractions
20190715 E. Strand
Testing the soft and hard homogenization protocol on extra molecular samples from the December recovery time period of the Holobiont Integration project. 2 M. capitata and 2 P. acuta fragments were randomly chosen from ATAC treatment 20181215.
| Extraction # | Coral ID | Species | Homogenization |
|---|---|---|---|
| 1 | 1123 | Montipora | Soft |
| 2 | 1123 | Montipora | Hard |
| 3 | 1769 | Montipora | Soft |
| 4 | 1769 | Montipora | Hard |
| 5 | 1056 | Pocillopora | Soft |
| 6 | 1056 | Pocillopora | Hard |
| 7 | 1607 | Pocillopora | Soft |
| 8 | 1607 | Pocillopora | Hard |
Fragment #1607 had algae covering the top of the fragment. Pieces were broken off at the bottom and used for the following extraction protocol.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet RNA DNA Extraction protocol found here.
2-3 fragment pieces were clipped per coral. The following photo depicts the fragment pieces in RNA DNA shield before soft and hard homogenization steps.
Pocillopora were “soft-homogenized” for 1 minute and Montipora for 2 minutes in a vortex. Both species were “hard-homogenized” for 30 seconds at 20 Hz in the Qiagen Tissue Lyser: Handbook.
Extraction notes:
- New Proteinase K made halfway through steps, new Proteinase K used for samples #6-8.
- Heat to lyse step start 10:30 end 12:00
- Extraction steps start 12:02 end ~14:00
Fragment pieces after the soft homogenization:
Fragment pieces after the hard homogenization:
DNA Master Mix Calculations:
- 75 μl DNA Digestion Buffer x 8 samples = 600 μl buffer
- 5 μl DNase I x 8 samples = 40 μl DNase I enzyme
Qubit Master Mix Calculations:
- 8 samples + 2 standards + 0.2% error = 10.2 μl Quant-IT reagent
- 199 * 10.2 = 2,029.8 μl buffer
Qubit (ng/μl) Results:
| DNA | |||
|---|---|---|---|
| Standard 1 | 239.46 | ||
| Standard 2 | 23085.86 | ||
| 1 | 1132 Mcap soft | 12.5 | 12.4 |
| 2 | 1132 Mcap hard | too low | too low |
| 3 | 1769 Mcap soft | 5.32 | 5.24 |
| 4 | 1769 Mcap hard | 4 | 3.9 |
| 5 | 1056 Pacuta soft | 9.74 | 9.5 |
| 6 | 1056 Pacuta hard | too low | too low |
| 7 | 1607 Pacuta soft | 4.18 | 4.12 |
| 8 | 1607 Pacuta hard | too low | too low |
| RNA | |||
|---|---|---|---|
| Standard 1 | 390.27 | ||
| Standard 2 | 10287.94 | ||
| 1 | 1132 Mcap soft | 38.4 | 38.2 |
| 2 | 1132 Mcap hard | 18.2 | 18.2 |
| 3 | 1769 Mcap soft | 44.6 | 44.4 |
| 4 | 1769 Mcap hard | 26 | 26.2 |
| 5 | 1056 Pacuta soft | 73.8 | 73.6 |
| 6 | 1056 Pacuta hard | 44.2 | 44 |
| 7 | 1607 Pacuta soft | 79 | 79 |
| 8 | 1607 Pacuta hard | 45.4 | 45.4 |
The value for DNA Standard 2 looks too high. Standards and Qubit re-done.
Qubit (ng/μl) round 2 results:
| DNA | |||
|---|---|---|---|
| Standard 1 | 152.27 | ||
| Standard 2 | 10492.87 | ||
| 1 | 1132 Mcap soft | 19 | 18.7 |
| 2 | 1132 Mcap hard | 6.8 | 6.74 |
| 3 | 1769 Mcap soft | 11 | 10.9 |
| 4 | 1769 Mcap hard | 9.24 | 9.12 |
| 5 | 1056 Pacuta soft | 17.9 | 17.5 |
| 6 | 1056 Pacuta hard | 2.96 | 2.72 |
| 7 | 1607 Pacuta soft | 8.62 | 8.42 |
| 8 | 1607 Pacuta hard | 3.18 | 3.22 |
| RNA | |||
|---|---|---|---|
| Standard 1 | 393.13 | ||
| Standard 2 | 10255.01 | ||
| 1 | 1132 Mcap soft | 41.2 | 41.2 |
| 2 | 1132 Mcap hard | 26.4 | 26.2 |
| 3 | 1769 Mcap soft | 48.2 | 48.2 |
| 4 | 1769 Mcap hard | 26 | 25.8 |
| 5 | 1056 Pacuta soft | 77 | 77 |
| 6 | 1056 Pacuta hard | 51 | 51 |
| 7 | 1607 Pacuta soft | 83.4 | 83.2 |
| 8 | 1607 Pacuta hard | 48 | 48 |
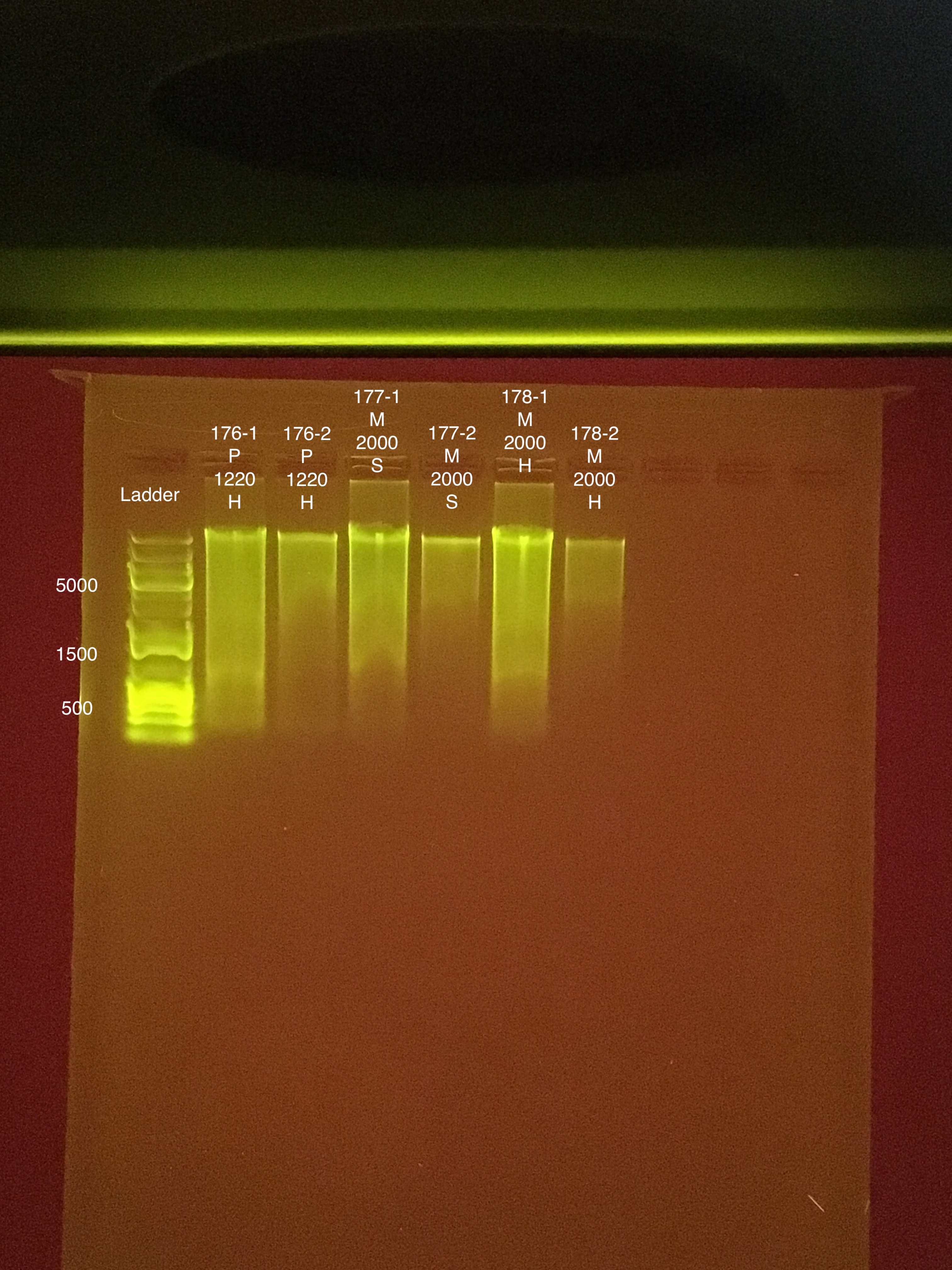
- 100V for 45 minutes, start 15:52 end 16:27
- 75 μl TAE buffer + 0.75 g agarose + 5 μl of Biotium gel RED gel stain to make a 1.5% gel
- 5 μl samples + 1 μl 6x purple loading dye
- 4 μl GeneRuler 1 kb Plus DNA ladder + 1 μl 6x purple loading dye
Sample order: Ladder, #1-8
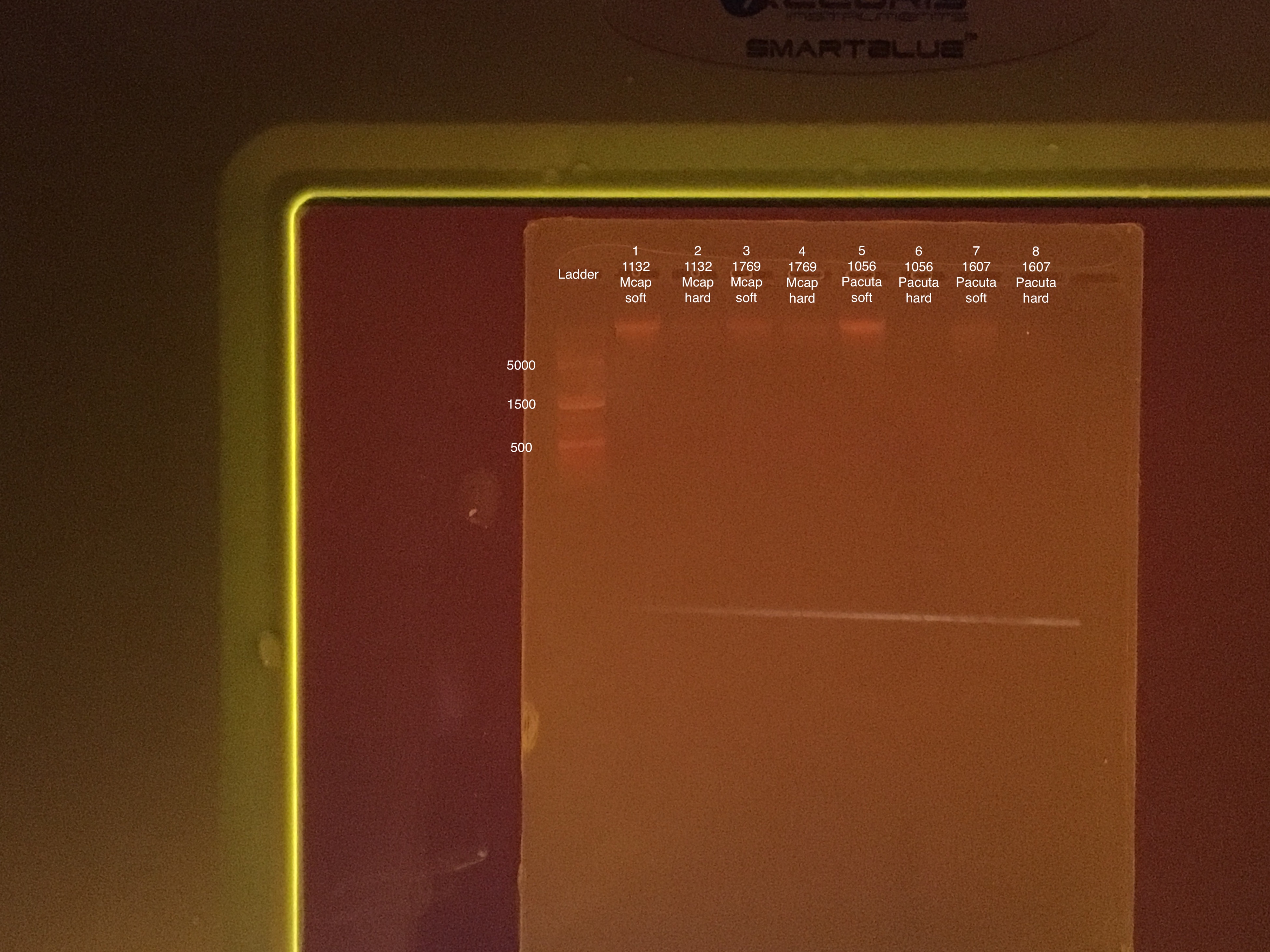
TapeStation Results:
| Extraction ID | Coral ID | RIN^e |
|---|---|---|
| 1 | 1132 Mcap soft | 5.8 |
| 2 | 1132 Mcap hard | 4.8 |
| 3 | 1769 Mcap soft | 6.8 |
| 4 | 1769 Mcap hard | 5.3 |
| 5 | 1056 Pacuta soft | 6.1 |
| 6 | 1056 Pacuta hard | 5 |
| 7 | 1607 Pacuta soft | 5 |
| 8 | 1607 Pacuta hard | 4.3 |
Link to the full 20190715 report Link to Agilent 4200 TapeStation System
- Thermocycler (rna denature program): 3 minutes at 72 °C, 2 minutes at 4 °C, hold at 4 °C.
- TapeStation start 16:17 end 16:27
- Ladder: Agilent RNA screentape ladder
20190716 E. Strand
Testing the soft and hard homogenization protocol on extra molecular samples from the December recovery time period of the Holobiont Integration project. 2 M. capitata and 2 P. acuta fragments were randomly chosen from ATAC treatment 20181215. These four fragments were used on 20190715 and 20190716.
| Extraction # | Coral ID | Species | Homogenization |
|---|---|---|---|
| 1 | 1123 | Montipora | Soft |
| 2 | 1123 | Montipora | Hard |
| 3 | 1769 | Montipora | Soft |
| 4 | 1769 | Montipora | Hard |
| 5 | 1056 | Pocillopora | Soft |
| 6 | 1056 | Pocillopora | Hard |
| 7 | 1607 | Pocillopora | Soft |
| 8 | 1607 | Pocillopora | Hard |
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet RNA DNA Extraction protocol found here.
With the following changes:
- No heat to lyse step to test if the heating is contributing to the RNA degradation.
- Just 1 fragment clipping instead of 2-3 clippings to test if the RNA/DNA shield is overloaded with more than one clipping.
- Diluted soft homogenization supernatant with 500 µl of RNA/DNA shield accidentally. Results suggest that this didn’t impact the quality, but may have diluted quantity.
Before homogenization steps:
After soft homogenization:
After hard homogenization:
Extraction steps: start 10:00 end 12:06. 8 samples takes about two hours.
1056 soft DNA final tube contains ~70-80 µl instead of 90 µl.
DNAse I Master Mix Calculations:
- 75 µl x 8 samples = 600 µl buffer
- 5 µl DNase I x 8 samples = 40 µl DNase I enzyme
Qubit Results:
Master Mix Calculations:
- 8 samples + 2 standards + 0.2% error = 10.2 µl Quant-IT Reagent
- 199 * 10.2 = 2,029.8 µl Qubit buffer
| DNA (ng/μl) | |||
|---|---|---|---|
| Standard 1 | 176.27 | ||
| Standard 2 | 20135.99 | ||
| 1 | 1132 Mcap soft | 20.8 | 20.8 |
| 2 | 1132 Mcap hard | 20.8 | 20.6 |
| 3 | 1769 Mcap soft | 27 | 26.8 |
| 4 | 1769 Mcap hard | 28 | 28 |
| 5 | 1056 Pacuta soft | 39.4 | 39.4 |
| 6 | 1056 Pacuta hard | 54 | 53.8 |
| 7 | 1607 Pacuta soft | 18.6 | 18.5 |
| 8 | 1607 Pacuta hard | 17.4 | 17.3 |
| RNA (ng/μl) | |||
|---|---|---|---|
| Standard 1 | 393.16 | ||
| Standard 2 | 10592.52 | ||
| 1 | 1132 Mcap soft | 11.8 | 11.8 |
| 2 | 1132 Mcap hard | 10.6 | 10.6 |
| 3 | 1769 Mcap soft | 16.6 | 16.6 |
| 4 | 1769 Mcap hard | 20.4 | 20.4 |
| 5 | 1056 Pacuta soft | 34.2 | 34.2 |
| 6 | 1056 Pacuta hard | 47.2 | 47.2 |
| 7 | 1607 Pacuta soft | 27.8 | 27.8 |
| 8 | 1607 Pacuta hard | 22.8 | 22.8 |
If the quantity is below 10 ng/μl in the soft homogenization, then re-extract DNA and RNA to allow for enough DNA/RNA for methylation and transcriptomic protocols/sequencing.
- 100V for 45 minutes, start 12:47 end 13:32
- 75 μl TAE buffer + 0.75 g agarose + 5 μl of Biotium gel RED gel stain to make a 1.5% gel.
- 5 μl samples + 1 μl 6x purple loading dye
- 4 μl GeneRuler 1 kb Plus DNA ladder + 1 μl 6x purple loading dye
Gel order: Ladder, 1-8
Ordered Biotium gel GREEN gel stain to use in the future instead of Biotium gel RED gel stain. Gel GREEN stain has been brighter in gels before for labmates.
TapeStation Results:
- Thermocycler (rna denature program): 3 minutes at 72 °C, 2 minutes at 4 °C, hold at 4 °C.
- TapeStation start 14:10 end 14:20
- Ladder: Agilent RNA screentape ladder
| Extraction ID | Coral ID | RIN^e |
|---|---|---|
| 1 | 1132 Mcap soft | 6.1 |
| 2 | 1132 Mcap hard | ** |
| 3 | 1769 Mcap soft | 7.7 |
| 4 | 1769 Mcap hard | 8.2 |
| 5 | 1056 Pacuta soft | 6.9 |
| 6 | 1056 Pacuta hard | 7.1 |
| 7 | 1607 Pacuta soft | 6.3 |
| 8 | 1607 Pacuta hard | 6.0 |
** Concentration too low for TapeStation to detect. But two small peaks are still visible in both reports. TapeStation done twice because samples were out of range. Not necessary in the future since a Qubit result of equal to or less than 10 ng/µl will likely read as too low of a concentration.
Link to the first 20190716 report
Link to the second 20190716 report
Link to Agilent 4200 TapeStation System
Future changes for next “round” of testing this protocol:
- Change hard homogenization in Tissue Lyser step to 1 minute instead of 30 seconds.
20190718 E.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here.
With the following changes:
- 1 minute hard homogenization step
Coral fragments were randomly chosen from timepoint bags.
| Timepoint | Coral Fragment ID | Species | Extraction ID | Homogenization |
|---|---|---|---|---|
| 20181117 | 1754 | Montipora capitata | 1 | Soft |
| 20181117 | 1754 | Montipora capitata | 2 | Hard |
| 20180922 | 1775 | Pocillopora acuta | 3 | Soft |
| 20180922 | 1775 | Pocillopora acuta | 4 | Hard |
| 20180922 | 2005 | Pocillopora acuta | 5 | Soft |
| 20180922 | 2005 | Pocillopora acuta | 6 | Hard |
| 20181020 | 2386 | Montipora capitata | 7 | Soft |
| 20181020 | 2386 | Montipora capitata | 8 | Hard |
| 20181103 | 2357 | Pocillopora acuta | 9 | Soft |
| 20181103 | 2357 | Pocillopora acuta | 10 | Hard |
| 20181103 | 1131 | Pocillopora acuta | 11 | Soft |
| 20181103 | 1131 | Pocillopora acuta | 12 | Hard |
| 20180922 | 2026 | Pocillopora acuta | 13 | Soft |
| 20180922 | 2026 | Pocillopora acuta | 14 | Hard |
| 20180922 | 1471 | Pocillopora acuta | 15 | Soft |
| 20180922 | 1471 | Pocillopora acuta | 16 | Hard |
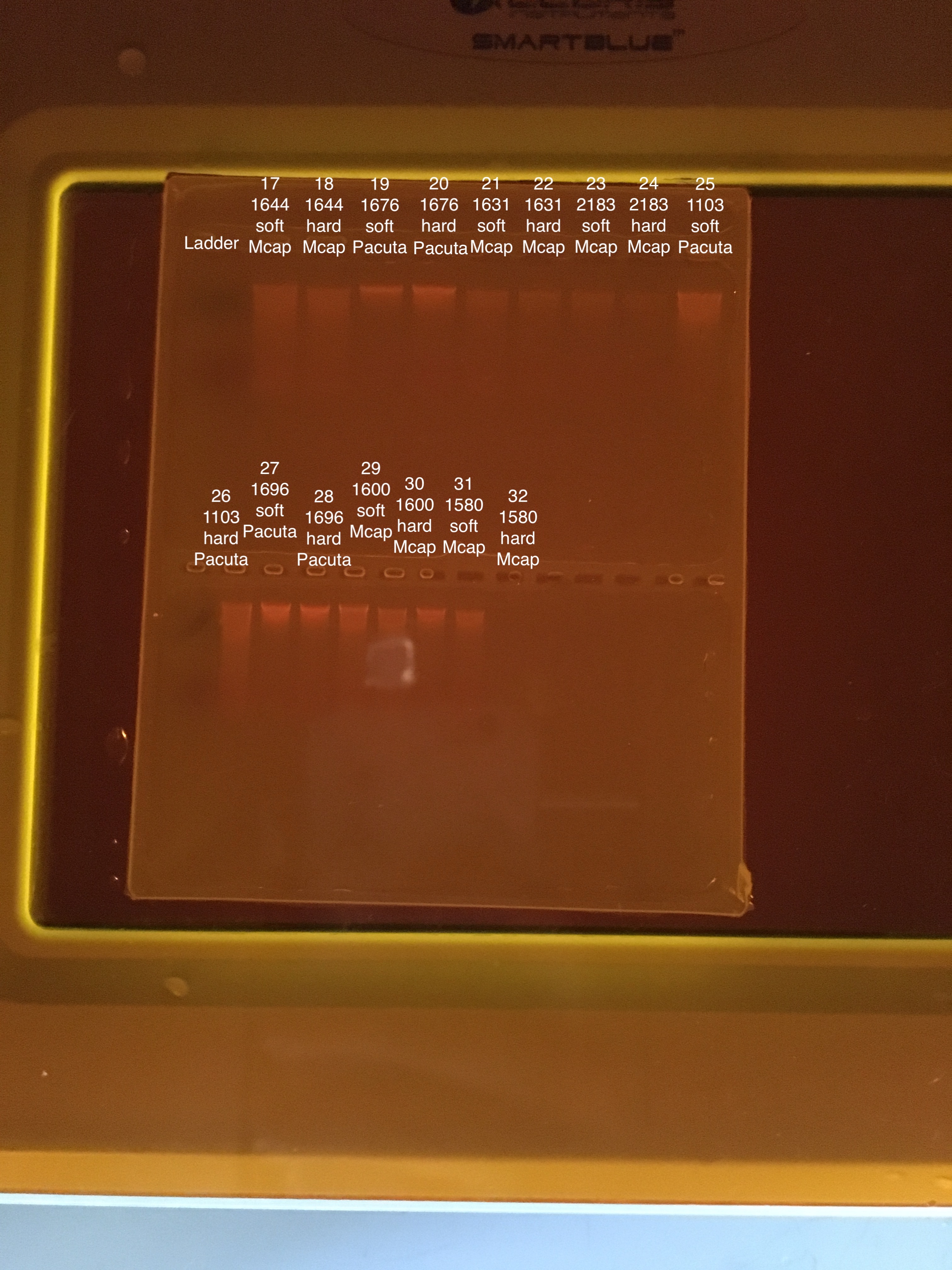
| 20181215 | 1644 | Montipora capitata | 17 | Soft |
| 20181215 | 1644 | Montipora capitata | 18 | Hard |
| 20180922 | 1676 | Pocillopora acuta | 19 | Soft |
| 20180922 | 1676 | Pocillopora acuta | 20 | Hard |
| 20181117 | 1631 | Montipora capitata | 21 | Soft |
| 20181117 | 1631 | Montipora capitata | 22 | Hard |
| 20180922 | 2183 | Montipora capitata | 23 | Soft |
| 20180922 | 2183 | Montipora capitata | 24 | Hard |
| 20181215 | 1103 | Pocillopora acuta | 25 | Soft |
| 20181215 | 1103 | Pocillopora acuta | 26 | Hard |
| 20181103 | 1696 | Pocillopora acuta | 27 | Soft |
| 20181103 | 1696 | Pocillopora acuta | 28 | Hard |
| 20180922 | 1600 | Montipora capitata | 29 | Soft |
| 20180922 | 1600 | Montipora capitata | 30 | Hard |
| 20181103 | 1580 | Montipora capitata | 31 | Soft |
| 20181103 | 1580 | Montipora capitata | 32 | Hard |
The 2 ATAC December timepoint coral fragments (1 Pocillopora, 1 Montipora) will be used for DNA methylation protocol testing.
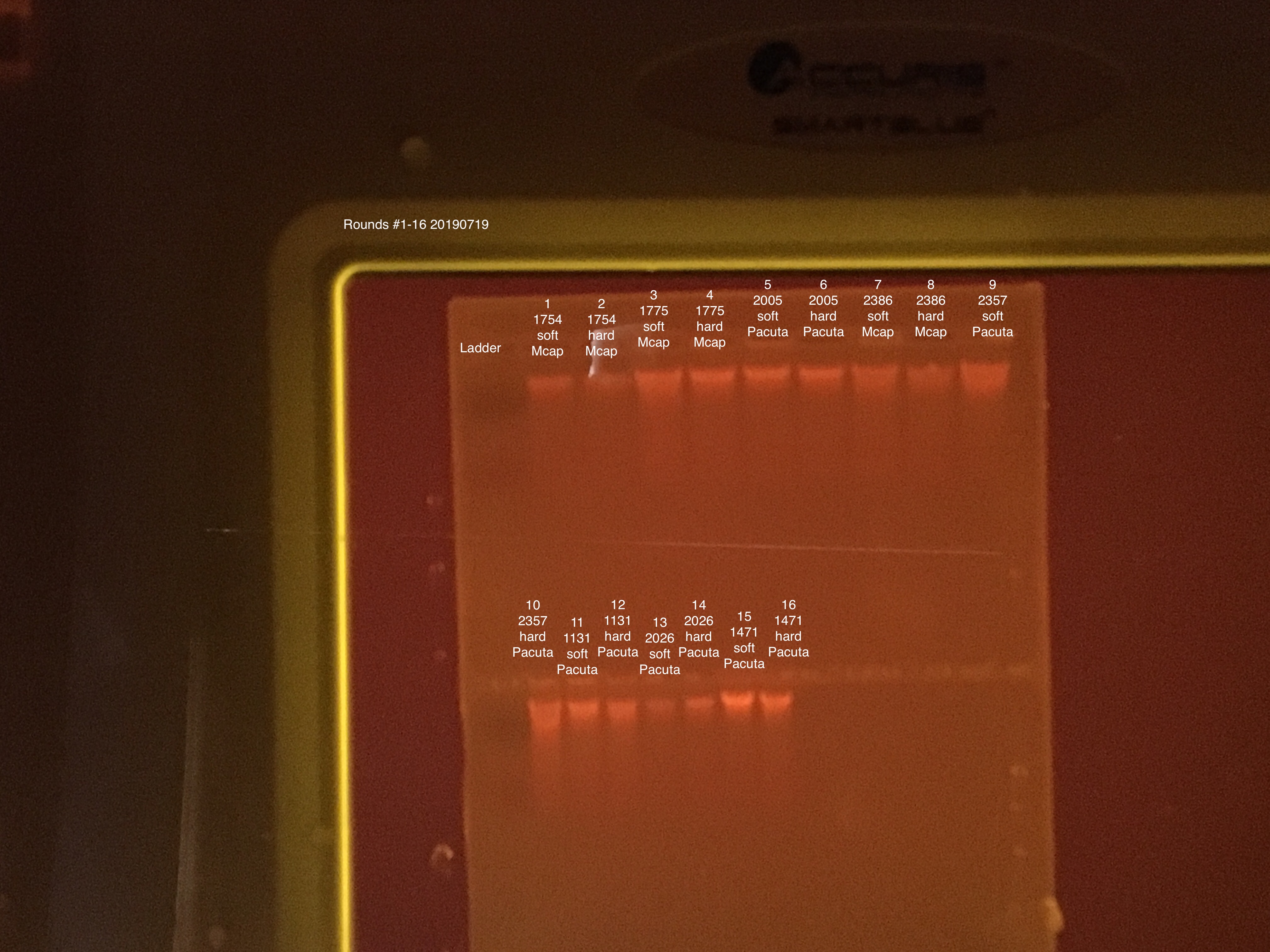
Extractions #1-16 done together and #17-32 done together.
Extractions #1-16:
Fragment Prep - start 10:00 end 10:42
Soft/Hard homogenization - start 10:43 end 11:22
Extractions - start 11:23 end 14:30
New DNase I Stock made and used for all samples.
Extractions #17-32:
Fragment Prep - start 14:30 end 15:00
Soft/Hard homogenization - start 15:00 end 15:37
Extractions - start 15:37 end 18:25
DNAse I Master Mix was not enough for all samples. New DNase I master mix made for last 2 samples.
Extraction ID #19 has 150 μl final volume.
20190719 E.S.
Gel electrophoresis, Qubit, and TapeStation from extractions #1-32.
Qubit Results:
| DNA | |||
|---|---|---|---|
| Standard 1 | 101.91 | ||
| Standard 2 | 8942.34 | ||
| Reading 1 | Reading 2 | Average | |
| 1 | 28 | 27.6 | 27.8 |
| 2 | 17.1 | 17 | 17.05 |
| 3 | 46.4 | 46 | 46.2 |
| 4 | 37.4 | 37 | 37.2 |
| 5 | 42.6 | 42.4 | 42.5 |
| 6 | 30.4 | 30.2 | 30.3 |
| 7 | 52.2 | 51.8 | 52 |
| 8 | 36.2 | 36 | 36.1 |
| 9 | 57.4 | 57 | 57.2 |
| 10 | 62 | 61.6 | 61.8 |
| 11 | 35.2 | 35 | 35.1 |
| 12 | 30.6 | 30.4 | 30.5 |
| 13 | 12 | 11.9 | 11.95 |
| 14 | 14.9 | 14.8 | 14.85 |
| 15 | 55 | 54.6 | 54.8 |
| 16 | 41.4 | 41 | 41.2 |
| 17 | 42.8 | 42.4 | 42.6 |
| 18 | 34.2 | 34 | 34.1 |
| 19 | 28.2 | 27.8 | 28 |
| 20 | 24 | 23 | 23.5 |
| 21 | 20.4 | 20.4 | 20.4 |
| 22 | 20.2 | 20.2 | 20.2 |
| 23 | 16.9 | 16.8 | 16.85 |
| 24 | 11.7 | 11.5 | 11.6 |
| 25 | 56.8 | 56.4 | 56.6 |
| 26 | 51 | 50.8 | 50.9 |
| 27 | 29 | 28.8 | 28.9 |
| 28 | 24 | 23.8 | 23.9 |
| 29 | 38.6 | 38.2 | 38.4 |
| 30 | 26.8 | 26.4 | 26.6 |
| 31 | 22.4 | 22.4 | 22.4 |
| 32 | 15.9 | 15.7 | 15.8 |
| RNA | |||
|---|---|---|---|
| Standard 1 | 399.52 | ||
| Standard 2 | 9930.43 | ||
| Reading 1 | Reading 2 | Average | |
| 1 | 15.4 | 15.4 | 15.4 |
| 2 | 12.4 | 12.4 | 12.4 |
| 3 | 57.8 | 57.6 | 57.7 |
| 4 | 46.2 | 46.2 | 46.2 |
| 5 | 41.2 | 41.2 | 41.2 |
| 6 | 37.8 | 37.8 | 37.8 |
| 7 | 36.4 | 36.4 | 36.4 |
| 8 | 31.4 | 31.4 | 31.4 |
| 9 | 78 | 77.8 | 77.9 |
| 10 | 48.4 | 48.4 | 48.4 |
| 11 | 54.8 | 54.8 | 54.8 |
| 12 | 48.6 | 48.4 | 48.5 |
| 13 | 23.2 | 23 | 23.1 |
| 14 | 27.4 | 27.4 | 27.4 |
| 15 | 54 | 53.8 | 53.9 |
| 16 | 53 | 52.8 | 52.9 |
| 17 | 16.8 | 16.8 | 16.8 |
| 18 | 15.8 | 15.8 | 15.8 |
| 19 | 41 | 41 | 41 |
| 20 | 37.2 | 37.2 | 37.2 |
| 21 | 13 | 13 | 13 |
| 22 | 13.4 | 13.8 | 13.6 |
| 23 | 13 | 12.8 | 12.9 |
| 24 | 10.8 | 10.6 | 10.7 |
| 25 | 62.6 | 62.6 | 62.6 |
| 26 | 38.4 | 38.2 | 38.3 |
| 27 | 40.2 | 40.2 | 40.2 |
| 28 | 33.6 | 33.6 | 33.6 |
| 29 | 14.8 | 14.8 | 14.8 |
| 30 | 15.2 | 15.2 | 15.2 |
| 31 | 24 | 24 | 24 |
| 32 | 11.8 | 11.8 | 11.8 |
Extractions #1-16:
Extractions #17-32:
TapeStation Results:
| Coral Fragment ID | Extraction ID | Homogenization | RIN |
|---|---|---|---|
| 1754 | 1 | Soft | 8 |
| 1754 | 2 | Hard | 7.7 |
| 1775 | 3 | Soft | 6.8 |
| 1775 | 4 | Hard | 6.9 |
| 2005 | 5 | Soft | 8.2 |
| 2005 | 6 | Hard | 8.1 |
| 2386 | 7 | Soft | 8.2 |
| 2386 | 8 | Hard | 7.8 |
| 2357 | 9 | Soft | 7.8 |
| 2357 | 10 | Hard | 7.3 |
| 1131 | 11 | Soft | 8 |
| 1131 | 12 | Hard | 7.7 |
| 2026 | 13 | Soft | 7.9 |
| 2026 | 14 | Hard | 7.6 |
| 1471 | 15 | Soft | 8.5 |
| 1471 | 16 | Hard | 8 |
| 1644 | 17 | Soft | 8.1 |
| 1644 | 18 | Hard | 8.2 |
| 1676 | 19 | Soft | 7.6 |
| 1676 | 20 | Hard | 7.3 |
| 1631 | 21 | Soft | 8.7 |
| 1631 | 22 | Hard | 8.7 |
| 2183 | 23 | Soft | 8.9 |
| 2183 | 24 | Hard | 8.9 |
| 1103 | 25 | Soft | 8.2 |
| 1103 | 26 | Hard | 7.9 |
| 1696 | 27 | Soft | ** |
| 1696 | 28 | Hard | 8.5 |
| 1600 | 29 | Soft | 9 |
| 1600 | 30 | Hard | 9 |
| 1580 | 31 | Soft | 8.7 |
| 1580 | 32 | Hard | 8.4 |
Link to 20190719 report, Extractions #1-15
Link to 20190719 report, Extractions #16-30
Link to 20190719 report, Extractions #31-32
Link to Agilent 4200 TapeStation System
Gel electrophoresis, qubit, and TapeStation for 16 samples (32 DNA extractions, 32 RNA extractions) takes ~4 hours.
20190720 E.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here.
Coral fragments were randomly chosen from different timepoint bags.
| Timepoint | Species | Coral ID | Extraction ID | Homogenization |
|---|---|---|---|---|
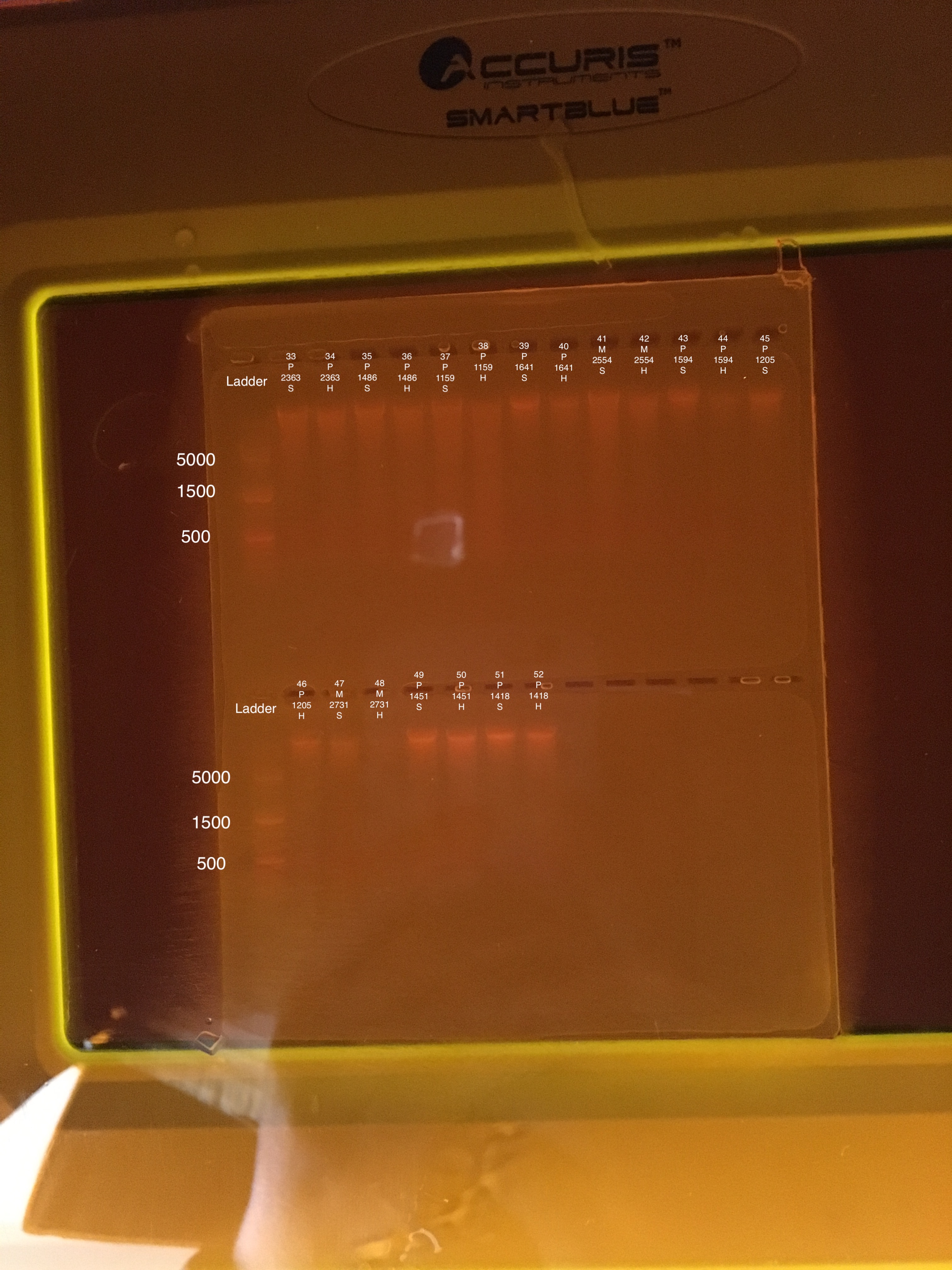
| 20180922 | Pocillopora | 2363 | 33 | Soft |
| 20180922 | Pocillopora | 2363 | 34 | Hard |
| 20181103 | Pocillopora | 1486 | 35 | Soft |
| 20181103 | Pocillopora | 1486 | 36 | Hard |
| 20181117 | Pocillopora | 1159 | 37 | Soft |
| 20181117 | Pocillopora | 1159 | 38 | Hard |
| 20181117 | Pocillopora | 1641 | 39 | Soft |
| 20181117 | Pocillopora | 1641 | 40 | Hard |
| 20181117 | Montipora | 2554 | 41 | Soft |
| 20181117 | Montipora | 2554 | 42 | Hard |
| 20181103 | Pocillopora | 1594 | 43 | Soft |
| 20181103 | Pocillopora | 1594 | 44 | Hard |
| 20181117 | Pocillopora | 1205 | 45 | Soft |
| 20181117 | Pocillopora | 1205 | 46 | Hard |
| 20180922 | Montipora | 2731 | 47 | Soft |
| 20180922 | Montipora | 2731 | 48 | Hard |
| 20181103 | Pocillopora | 1451 | 49 | Soft |
| 20181103 | Pocillopora | 1451 | 50 | Hard |
| 20180922 | Pocillopora | 1418 | 51 | Soft |
| 20180922 | Pocillopora | 1418 | 52 | Hard |
Fragment Preparation start 9:08 end 9:46
Soft/Hard homogenizations start 9:46 end 10:31
Extractions start 10:31 end 13:35
~4.5 hours for 10 coral fragments (20 DNA, 20 RNA Extractions)
Qubit Results:
| DNA (ng/μl) | ||||
|---|---|---|---|---|
| Standard 1 | 181.64 | |||
| Standard 2 | 21322.7 | |||
| Coral ID | Extraction ID | Reading 1 | Reading 2 | Average |
| 2363 | 33 | 57.2 | 57.2 | 57.2 |
| 2363 | 34 | 40.4 | 40 | 40.2 |
| 1486 | 35 | 43 | 42.8 | 42.9 |
| 1486 | 36 | 37.2 | 37.2 | 37.2 |
| 1159 | 37 | 53.2 | 53 | 53.1 |
| 1159 | 38 | 4.4 | 4.36 | 4.38 |
| 1641 | 39 | 34.6 | 34 | 34.3 |
| 1641 | 40 | 27 | 26.8 | 26.9 |
| 2554 | 41 | 58.4 | 58.2 | 58.3 |
| 2554 | 42 | 31.2 | 31.2 | 31.2 |
| 1594 | 43 | 28.2 | 28 | 28.1 |
| 1594 | 44 | 16.1 | 16.1 | 16.1 |
| 1205 | 45 | 34.6 | 34.6 | 34.6 |
| 1205 | 46 | 27.4 | 27.2 | 27.3 |
| 2731 | 47 | 27.4 | 27.4 | 27.4 |
| 2731 | 48 | 12.9 | 12.8 | 12.85 |
| 1451 | 49 | 39.4 | 39.2 | 39.3 |
| 1451 | 50 | 39.6 | 39.4 | 39.5 |
| 1418 | 51 | 32 | 31.8 | 31.9 |
| 1418 | 52 | 31.2 | 31.2 | 31.2 |
| RNA (ng/μl) | ||||
|---|---|---|---|---|
| Standard 1 | 397.95 | |||
| Standard 2 | 10082.2 | |||
| Coral ID | Extraction ID | Reading 1 | Reading 2 | Average |
| 2363 | 33 | 69.4 | 69.4 | 69.4 |
| 2363 | 34 | 48 | 48 | 48 |
| 1486 | 35 | 82.6 | 82.6 | 82.6 |
| 1486 | 36 | 48.2 | 48 | 48.1 |
| 1159 | 37 | 47 | 47 | 47 |
| 1159 | 38 | 45.2 | 45.2 | 45.2 |
| 1641 | 39 | 46.4 | 46.4 | 46.4 |
| 1641 | 40 | 26.6 | 26.6 | 26.6 |
| 2554 | 41 | 27.6 | 27.6 | 27.6 |
| 2554 | 42 | 23 | 23 | 23 |
| 1594 | 43 | 29.4 | 29.4 | 29.4 |
| 1594 | 44 | 25.8 | 25.8 | 25.8 |
| 1205 | 45 | 59.4 | 59.4 | 59.4 |
| 1205 | 46 | 45.4 | 45.4 | 45.4 |
| 2731 | 47 | 22.6 | 22.6 | 22.6 |
| 2731 | 48 | 17.2 | 17.2 | 17.2 |
| 1451 | 49 | 29 | 29 | 29 |
| 1451 | 50 | 45.8 | 45.6 | 45.7 |
| 1418 | 51 | 57.8 | 57.8 | 57.8 |
| 1418 | 52 | 45.4 | 45.4 | 45.4 |
TapeStation Results:
| Coral ID | Extraction ID | RIN |
|---|---|---|
| 2363 | 33 | 7.4 |
| 2363 | 34 | 7.5 |
| 1486 | 35 | 7.3 |
| 1486 | 36 | 7 |
| 1159 | 37 | 6.9 |
| 1159 | 38 | 6.5 |
| 1641 | 39 | 7.4 |
| 1641 | 40 | 6.8 |
| 2554 | 41 | 9.1 |
| 2554 | 42 | 8.6 |
| 1594 | 43 | 7.6 |
| 1594 | 44 | 7.8 |
| 1205 | 45 | 7.3 |
| 1205 | 46 | 7 |
| 2731 | 47 | 5.7 |
| 2731 | 48 | ** |
| 1451 | 49 | 7.7 |
| 1451 | 50 | 7.7 |
| 1418 | 51 | 7.4 |
| 1418 | 52 | 7 |
** Concentration too low to read.
Not enough buffer in samples #35,36. Values in second report.
Link to 20190720 report #1, Extractions #33-52
Link to 20190720 report #2, Extractions #33-52
Link to Agilent 4200 TapeStation System
20190722 and 20190723 E.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here.
Coral fragments were randomly chosen from different timepoint bags.
| Timepoint | Species | Coral ID | Extraction ID | Homgenization |
|---|---|---|---|---|
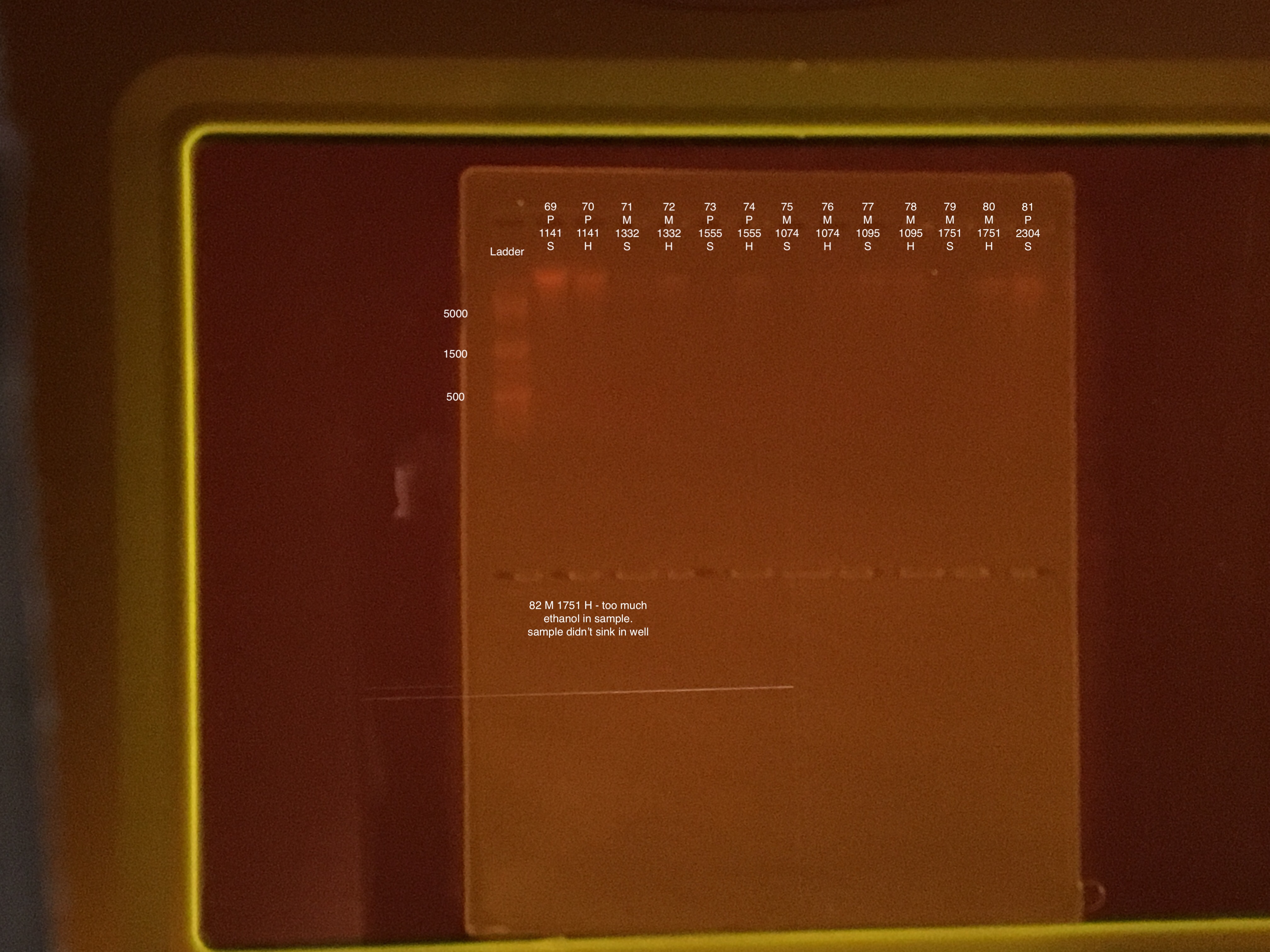
| 20181215 | Montipora | 1555 | 73 | soft |
| 20181215 | Montipora | 1555 | 74 | hard |
| 20181117 | Montipora | 1074 | 75 | soft |
| 20181117 | Montipora | 1074 | 76 | hard |
| 20181117 | Montipora | 1095 | 77 | soft |
| 20181117 | Montipora | 1095 | 78 | hard |
| 20180922 | Montipora | 1751 | 79 | soft |
| 20180922 | Montipora | 1751 | 80 | hard |
| 20181020 | Pocillopora | 2304 | 81 | soft |
| 20181020 | Pocillopora | 2304 | 82 | hard |
Fragment prep start 15:05 end 15:22
Soft/Hard homogenization start 15:23 end 15:48
Extractions start 15:49 end 17:53
50 μl of 100% ethanol added to extraction #s: 79, 73, 75, 82, and 81 in final elution step accidentally. Final volume of the above 5 DNA extractions is 150 μl. Residual ethanol will prevent full hydration and elution of nucleic acids, and too much ethanol will cause the sample to not sink into a well in the DNA gel. These five samples might be too diluted to quantify DNA.
Qubit Results:
| DNA (ng/μl) | ||||
|---|---|---|---|---|
| Standard 1 | 209.93 | |||
| Standard 2 | 23170.52 | |||
| Coral ID | Extraction ID | Reading 1 | Reading 2 | Average |
| 1555 | 73 | 4.58 | 4.54 | 4.56 |
| 1555 | 74 | 7.72 | 7.7 | 7.71 |
| 1074 | 75 | ** | ** | ** |
| DNA | ||||
| Standard 1 | 169.27 | |||
| Standard 2 | 18543.03 | |||
| Coral ID | Extraction ID | Reading 1 | Reading 2 | Average |
| 1074 | 76 | 5.38 | 5.28 | 5.33 |
| 1095 | 77 | 7.54 | 7.44 | 7.49 |
| 1095 | 78 | 8.32 | 8.28 | 8.3 |
| 1751 | 79 | 2.9 | 2.88 | 2.89 |
| 1751 | 80 | 8 | 7.96 | 7.98 |
| 2304 | 81 | 25.2 | 25 | 25.1 |
| 2304 | 82 | 12.8 | 12.7 | 12.75 |
| RNA (ng/μl) | ||||
|---|---|---|---|---|
| Standard 1 | 411.38 | |||
| Standard 2 | 12375.27 | |||
| Coral ID | Extraction ID | Reading 1 | Reading 2 | Average |
| 1555 | 73 | ** | ** | ** |
| 1555 | 74 | ** | ** | ** |
| 1074 | 75 | ** | ** | ** |
| 1074 | 76 | ** | ** | ** |
| 1095 | 77 | ** | ** | ** |
| 1095 | 78 | ** | ** | ** |
| 1751 | 79 | ** | ** | ** |
| 1751 | 80 | ** | ** | ** |
| 2304 | 81 | 24.4 | 24.4 | 24.4 |
| 2304 | 82 | 32.2 | 32.4 | 32.3 |
Because of the ethanol added to 5 of the DNA samples, 3 of the samples did not sink into the gel - #73, 75, and 82.
TapeStation Results:
| Coral Fragment ID | Extraction ID | RIN |
|---|---|---|
| 1555 | 73 | ** |
| 1555 | 74 | ** |
| 1074 | 75 | ** |
| 1074 | 76 | ** |
| 1095 | 77 | ** |
| 1095 | 78 | ** |
| 1751 | 79 | ** |
| 1751 | 80 | ** |
| 2304 | 81 | 9 |
| 2304 | 82 | 8.7 |
Link to 20190723 report #3, Extractions #68-82
Will re-do fragment #s: 1420, 1689, 1235, 1332, 1555, 1074, 1095, and 1751.
20190723 E.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here.
Coral fragments were randomly chosen from different timepoint bags.
| Timepoint | Species | Coral ID | Extraction ID | Homogenization |
|---|---|---|---|---|
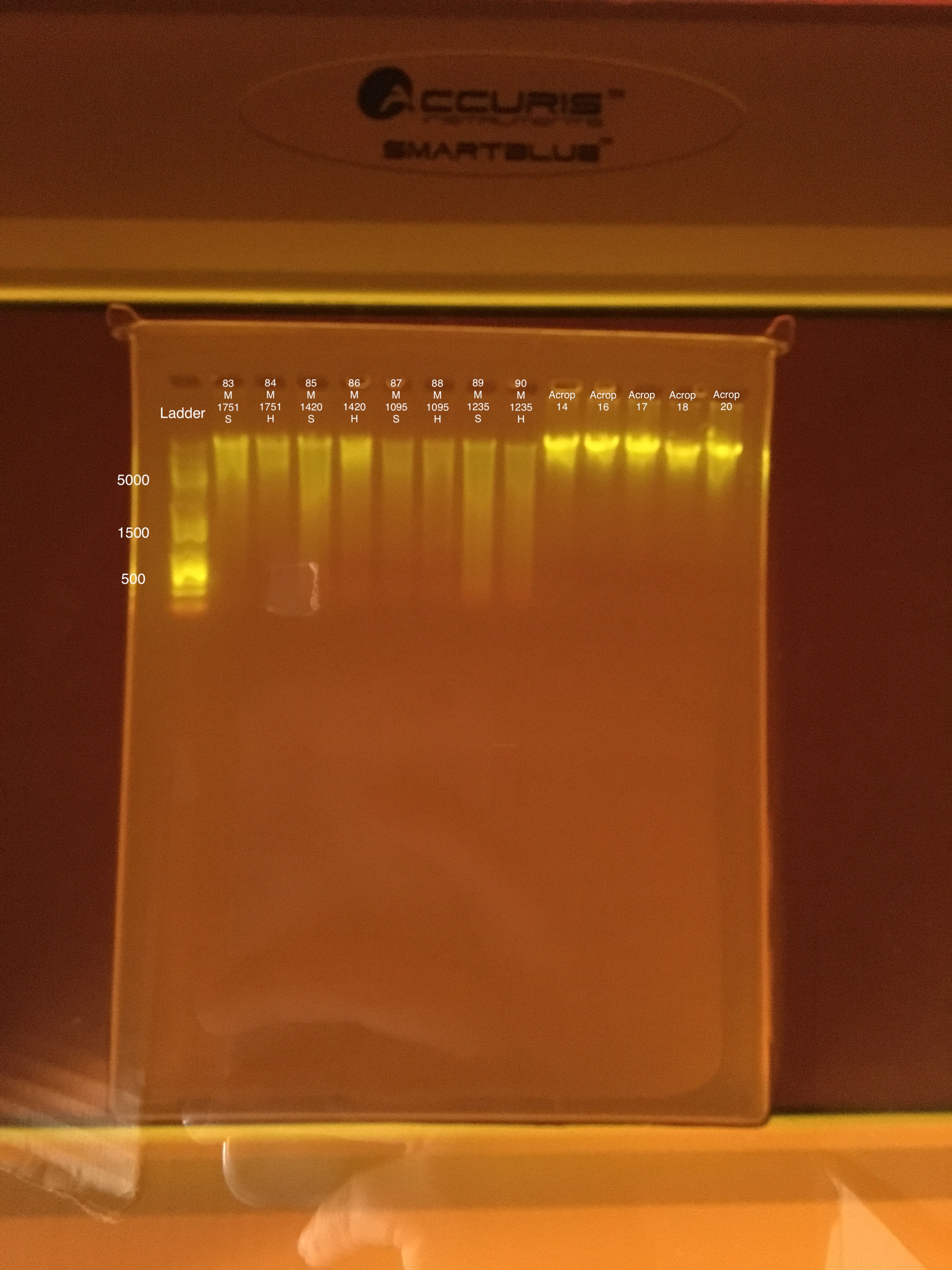
| 20180922 | Montipora | 1751 | 83 | soft |
| 20180922 | Montipora | 1751 | 84 | hard |
| 20181103 | Montipora | 1420 | 85 | soft |
| 20181103 | Montipora | 1420 | 86 | hard |
| 20181117 | Montipora | 1095 | 87 | soft |
| 20181117 | Montipora | 1095 | 88 | hard |
| 20181020 | Montipora | 1235 | 89 | soft |
| 20181020 | Montipora | 1235 | 90 | hard |
Fragment preparation start ~13:06 end 13:15
Soft/Hard homogenization end 13:45
Extractions end ~15:40
Qubit Results:
| DNA (ng/μl) | ||||
|---|---|---|---|---|
| Standard 1 | 179.71 | |||
| Standard 2 | 20560.79 | |||
| Coral ID | Extraction ID | Reading 1 | Reading 2 | Average |
| 1751 | 83 | 34.6 | 34.2 | 34.4 |
| 1751 | 84 | 18.3 | 18.1 | 18.2 |
| 1420 | 85 | 34.8 | 34.4 | 34.6 |
| 1420 | 86 | 21 | 20.8 | 20.9 |
| 1095 | 87 | 13.8 | 13.8 | 13.8 |
| 1095 | 88 | 12.9 | 12.9 | 12.9 |
| 1235 | 89 | 34.8 | 34.6 | 34.7 |
| 1235 | 90 | 15.1 | 15.1 | 15.1 |
| RNA (ng/μl) | ||||
|---|---|---|---|---|
| Standard 1 | 407.62 | |||
| Standard 2 | 10776.89 | |||
| Coral ID | Extraction ID | Reading 1 | Reading 2 | Average |
| 1751 | 83 | 17 | 16.8 | 16.9 |
| 1751 | 84 | 12.2 | 12.2 | 12.2 |
| 1420 | 85 | 37.2 | 37.2 | 37.2 |
| 1420 | 86 | 18.6 | 18.6 | 18.6 |
| 1095 | 87 | 18.6 | 15.8 | 17.2 |
| 1095 | 88 | 11.2 | 11 | 11.1 |
| 1235 | 89 | 23.2 | 23 | 23.1 |
| 1235 | 90 | 28.8 | 23.6 | 26.20 |
Extractions #: 83-90
TapeStation Results:
| Coral Fragment ID | Extraction ID | RIN |
|---|---|---|
| 1751 | 83 | 8.7 |
| 1751 | 84 | 8.6 |
| 1420 | 85 | 8.4 |
| 1420 | 86 | 8.5 |
| 1095 | 87 | 8.3 |
| 1095 | 88 | 8.4 |
| 1235 | 89 | 7.7 |
| 1235 | 90 | 7.9 |
Link to 20190723 report #3, Extractions #83-90
20190724 E.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here.
Coral fragments were randomly chosen from different timepoint bags.
Extractions #91-98 done together and extractions #99-118 done together.
Extractions #91-98 start 9:15 end 12:05
Extractions #99-118 start 12:15 end 16:36
| Timepoint | Species | Coral ID | Extraction ID | Homogenization |
|---|---|---|---|---|
| 20181117 | Montipora | 1332 | 91 | soft |
| 20181117 | Montipora | 1332 | 92 | hard |
| 20181117 | Montipora | 1074 | 93 | soft |
| 20181117 | Montipora | 1074 | 94 | hard |
| 20181103 | Montipora | 1420 | 95 | soft |
| 20181103 | Montipora | 1420 | 96 | hard |
| 20181215 | Montipora | 1555 | 97 | soft |
| 20181215 | Montipora | 1555 | 98 | hard |
| 20181103 | Montipora | 1148 | 99 | soft |
| 20181103 | Montipora | 1148 | 100 | hard |
| 20181103 | Pocillopora | 2202 | 101 | soft |
| 20181103 | Pocillopora | 2202 | 102 | hard |
| 20181103 | Pocillopora | 2305 | 103 | soft |
| 20181103 | Pocillopora | 2305 | 104 | hard |
| 20181103 | Montipora | 2862 | 105 | soft |
| 20181103 | Montipora | 2862 | 106 | hard |
| 20181020 | Montipora | 1260 | 107 | soft |
| 20181020 | Montipora | 1260 | 108 | hard |
| 20180922 | Montipora | 2561 | 109 | soft |
| 20180922 | Montipora | 2561 | 110 | hard |
| 20181117 | Pocillopora | 1732 | 111 | soft |
| 20181117 | Pocillopora | 1732 | 112 | hard |
| 20180922 | Pocillopora | 2743 | 113 | soft |
| 20180922 | Pocillopora | 2743 | 114 | hard |
| 20180922 | Pocillopora | 1219 | 115 | soft |
| 20180922 | Pocillopora | 1219 | 116 | hard |
| 20181117 | Pocillopora | 2550 | 117 | soft |
| 20181117 | Pocillopora | 2550 | 118 | hard |
Fragments #1074, #1732, and #2202 were paler in color and liquid looked less dense after soft and hard homogenizations.
Qubit Results:
| DNA (ng/μl) | ||
|---|---|---|
| Standard 1 | 215.12 | |
| Standard 2 | 23344.97 | |
| Coral ID | Extraction ID | Reading |
| 1332 | 91 | 35 |
| 1332 | 92 | 24 |
| 1074 | 93 | 41.2 |
| 1074 | 94 | 22.2 |
| 1420 | 95 | 49.8 |
| 1420 | 96 | 32.8 |
| 1555 | 97 | 51.4 |
| 1555 | 98 | 31.4 |
| DNA (ng/μl) | ||
|---|---|---|
| Standard 1 | 226.84 | |
| Standard 2 | 24638.56 | |
| Coral ID | Extraction ID | Reading |
| 1148 | 99 | 59.2 |
| 1148 | 100 | 28 |
| 2202 | 101 | 70.8 |
| 2202 | 102 | 50.8 |
| 2305 | 103 | 80.8 |
| 2305 | 104 | 83 |
| 2862 | 105 | 30.2 |
| 2862 | 106 | 24 |
| 1260 | 107 | 19.6 |
| 1260 | 108 | 11.9 |
| 2561 | 109 | 14.8 |
| 2561 | 110 | 16.9 |
| 1732 | 111 | 46.2 |
| 1732 | 112 | 42.6 |
| 2743 | 113 | 106 |
| 2743 | 114 | 101 |
| 1219 | 115 | 77.4 |
| 1219 | 116 | 40.2 |
| 2550 | 117 | 38.6 |
| 2550 | 118 | 60.4 |
| RNA (ng/μl) | ||
|---|---|---|
| Standard 1 | 413.78 | |
| Standard 2 | 11048.94 | |
| Coral ID | Extraction ID | Reading |
| 1332 | 91 | 31.2 |
| 1332 | 92 | 17.8 |
| 1074 | 93 | 14.8 |
| 1074 | 94 | 10.4 |
| 1420 | 95 | 30.2 |
| 1420 | 96 | 19.2 |
| 1555 | 97 | 31.2 |
| 1555 | 98 | 20.8 |
| RNA (ng/μl) | ||||
|---|---|---|---|---|
| Standard 1 | 408.66 | |||
| Standard 2 | 11009.82 | |||
| Coral ID | Extraction ID | Reading 1 | Reading 2 | Average |
| 1148 | 99 | 26.4 | 25.8 | 26.1 |
| 1148 | 100 | 15 | 15 | 15 |
| 2202 | 101 | 77.4 | 77.4 | 77.4 |
| 2202 | 102 | 50.8 | 50.8 | 50.8 |
| 2305 | 103 | 94.6 | 94.4 | 94.5 |
| 2305 | 104 | 63.2 | 63.2 | 63.2 |
| 2862 | 105 | 21 | 20.8 | 20.9 |
| 2862 | 106 | 14.2 | 14.2 | 14.2 |
| 1260 | 107 | 16.8 | 16.8 | 16.8 |
| 1260 | 108 | 11.2 | 11 | 11.1 |
| 2561 | 109 | 16.6 | 16.8 | 16.7 |
| 2561 | 110 | 12.4 | 12 | 12.2 |
| 1732 | 111 | 54.6 | 54.6 | 54.6 |
| 1732 | 112 | 37.2 | 37.2 | 37.2 |
| 2743 | 113 | 103 | 103 | 103 |
| 2743 | 114 | 69 | 69 | 69 |
| 1219 | 115 | 75.8 | 75.6 | 75.7 |
| 1219 | 116 | 53.2 | 52.8 | 53 |
| 2550 | 117 | 47 | 47 | 47 |
| 2550 | 118 | 42.8 | 42.8 | 42.8 |
Two readings were only done for RNA extractions #99-118.
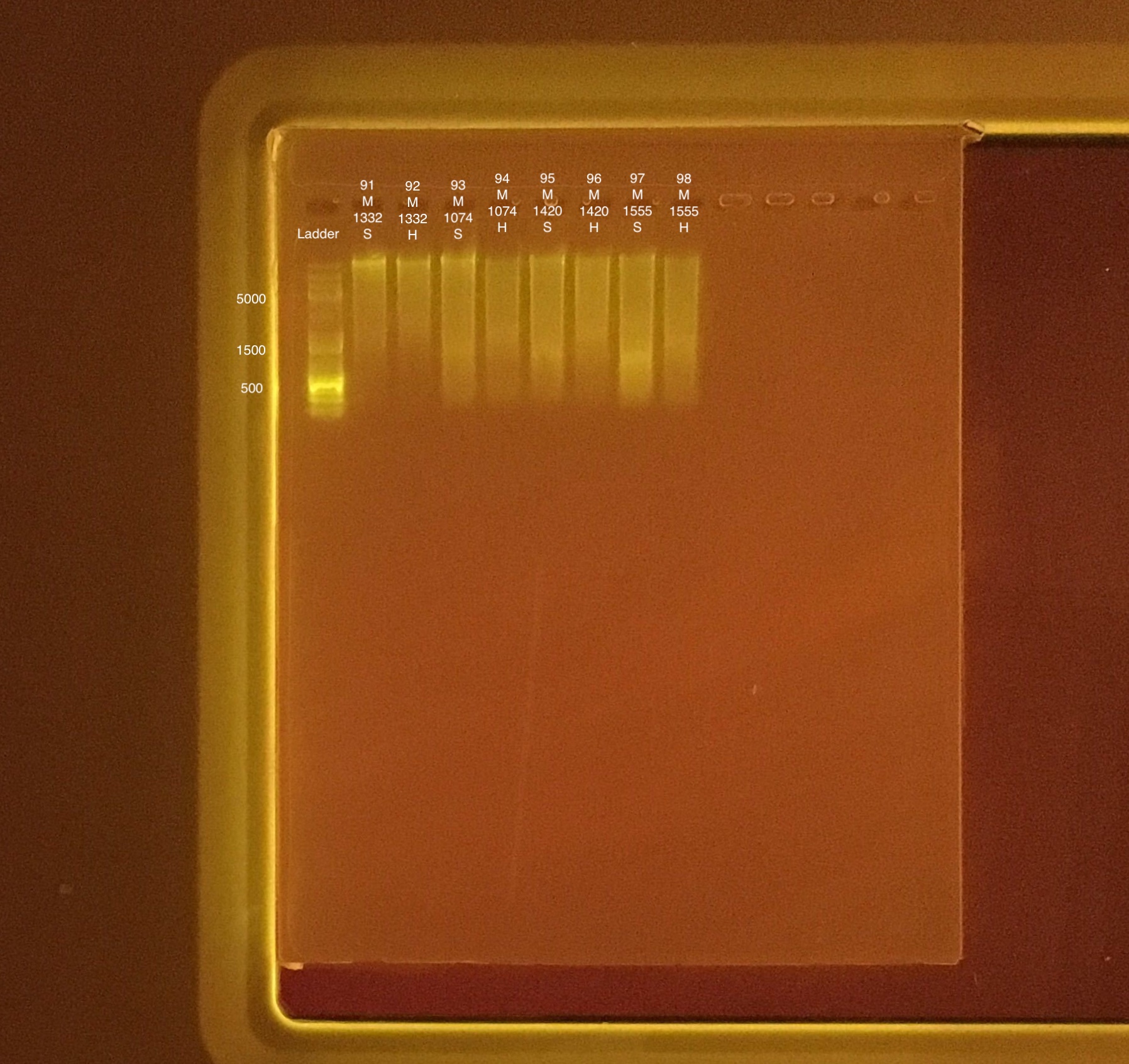
Extractions #91-98
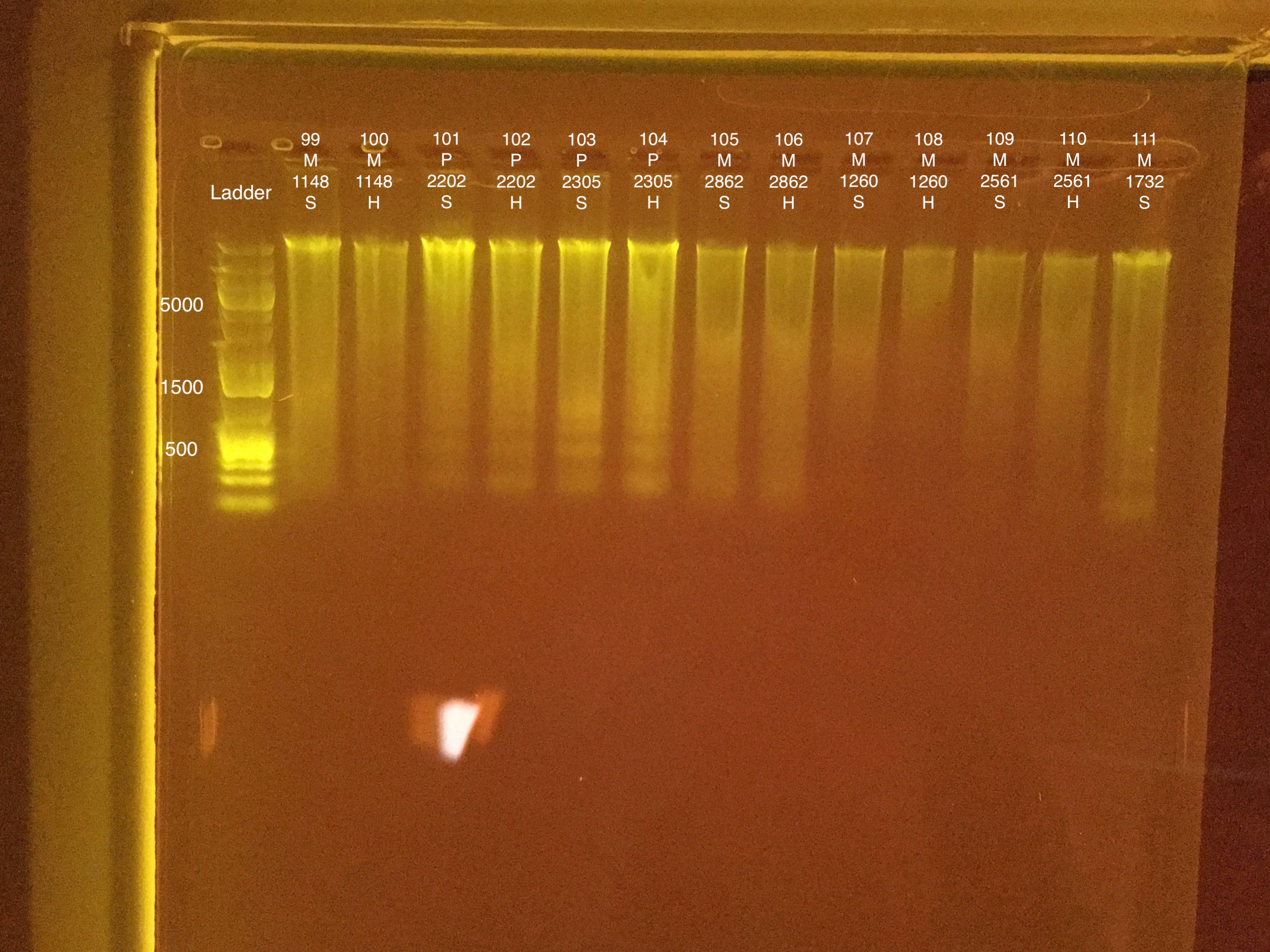
Extractions #99-111
Extractions #112-118
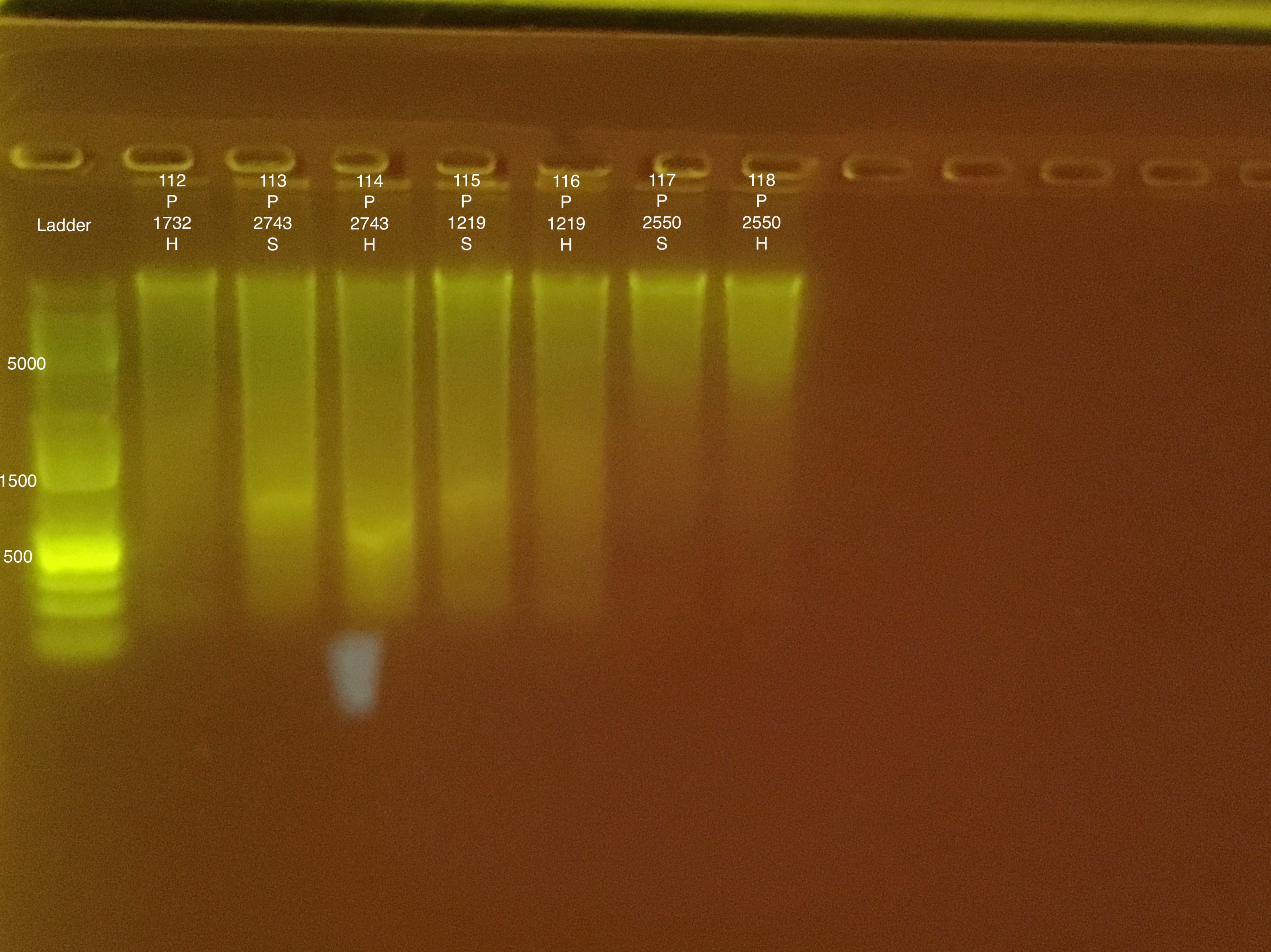
TapeStation Results:
| Coral ID | Extraction ID | RIN |
|---|---|---|
| 1332 | 91 | 7 |
| 1332 | 92 | 7.3 |
| 1074 | 93 | 8.2 |
| 1074 | 94 | ** |
| 1420 | 95 | 8.2 |
| 1420 | 96 | 8.3 |
| 1555 | 97 | 8.4 |
| 1555 | 98 | 8.2 |
| 1148 | 99 | 8.5 |
| 1148 | 100 | 8.5 |
| 2202 | 101 | 8.2 |
| 2202 | 102 | 7.6 |
| 2305 | 103 | 7.7 |
| 2305 | 104 | 7.7 |
| 2862 | 105 | 8.8 |
| 2862 | 106 | 8.9 |
| 1260 | 107 | 8.8 |
| 1260 | 108 | 8.1 |
| 2561 | 109 | 8 |
| 2561 | 110 | 8.2 |
| 1732 | 111 | 8.4 |
| 1732 | 112 | 8.1 |
| 2743 | 113 | 7 |
| 2743 | 114 | 6.5 |
| 1219 | 115 | 7.4 |
| 1219 | 116 | 7.4 |
| 2550 | 117 | 8.6 |
| 2550 | 118 | 8.3 |
TapeStation report #1 does not have descriptions. Order of samples: Ladder, 91-98.
Link to 20190724 report #1, Extractions #91-98
Link to 20190724 report #2, Extractions #99-118
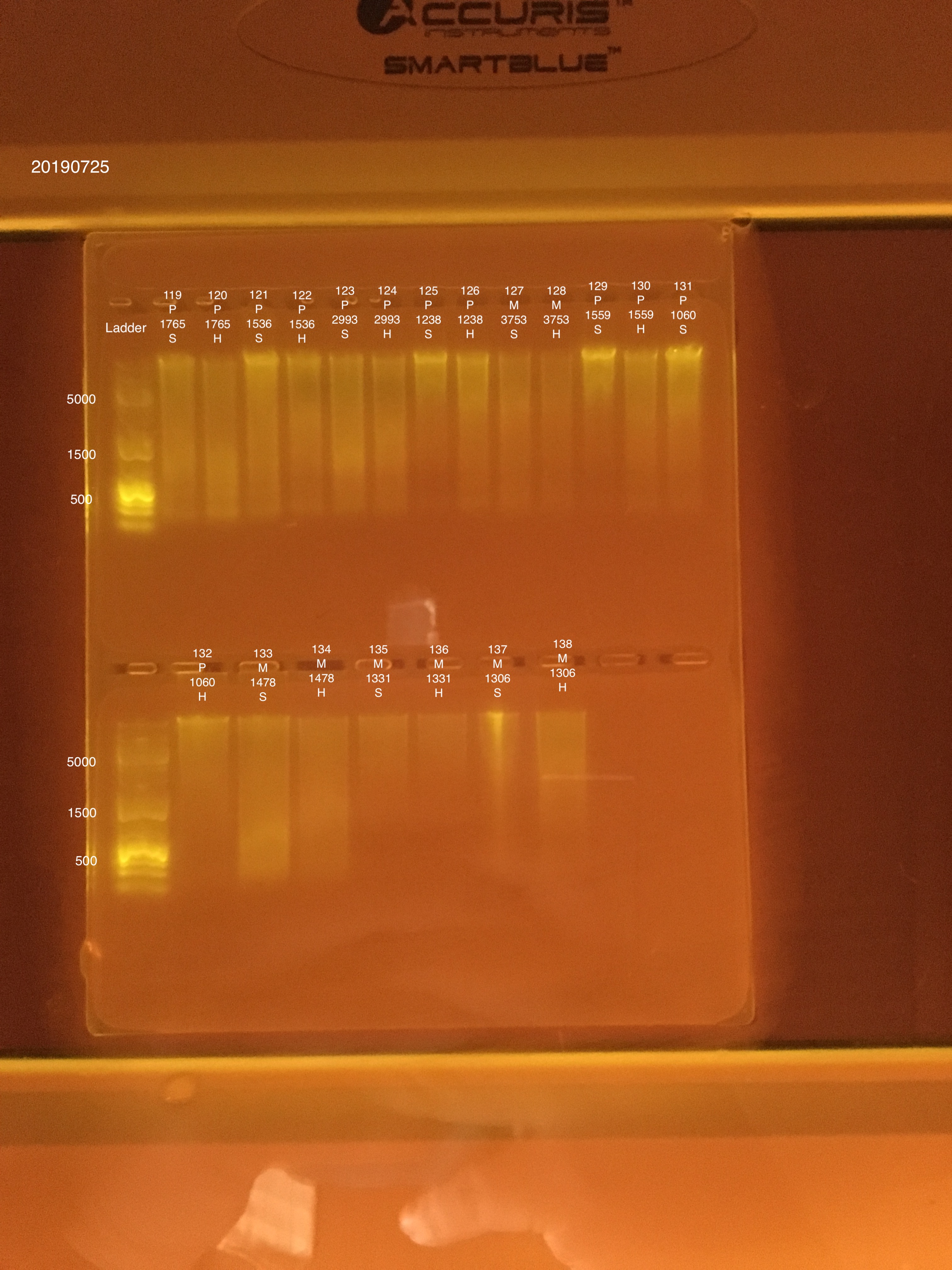
20190725 E.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here.
Coral fragments were randomly chosen from different timepoint bags.
Fragment preparation start ~9:20 end 9:50
Soft/Hard homogenization start 9:51 end 10:33
RNA DNA Extraction start 10:34 end ~13:30
Qubit used to check DNA and RNA quantity (ng/μl).
TapeStation used to check RNA quality.
DNA Standard 1: 215.21 ng/μl
DNA Standard 2: 24479.38 ng/μl
RNA Standard 1: 410.82 ng/μl
RNA Standard 2: 11192.82 ng/μl
| Timepoint | Species | Coral ID | Extraction ID | Homogenization | DNA Reading 1 | DNA Reading 2 | Average DNA ng/μl | RNA Reading 1 | RNA Reading 2 | Average RNA ng/μl | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20181020 | Pocillopora | 1765 | 119 | soft | 77.6 | 74.4 | 76 | 67.6 | 67 | 67.3 | 7.2 |
| 20181020 | Pocillopora | 1765 | 120 | hard | 51.4 | 49.6 | 50.5 | 37.6 | 37.2 | 37.4 | 6.7 |
| 20181117 | Pocillopora | 1536 | 121 | soft | 80 | 79 | 79.5 | 61.8 | 61.4 | 61.6 | 7.8 |
| 20181117 | Pocillopora | 1536 | 122 | hard | 58.4 | 58 | 58.2 | 49.4 | 48.8 | 49.1 | 7.6 |
| 20180922 | Pocillopora | 2993 | 123 | soft | 82.2 | 80.8 | 81.5 | 73.8 | 73.6 | 73.7 | 6.6 |
| 20180922 | Pocillopora | 2993 | 124 | hard | 48.6 | 48.2 | 48.4 | 49 | 48.8 | 48.9 | 6.3 |
| 20181117 | Pocillopora | 1238 | 125 | soft | 43.4 | 42.8 | 43.1 | 55.4 | 55 | 55.2 | 8.5 |
| 20181117 | Pocillopora | 1238 | 126 | hard | 27.6 | 27.2 | 27.4 | 31.8 | 31.6 | 31.7 | 8.1 |
| 20181020 | Montipora | 3753 | 127 | soft | 19.5 | 19.4 | 19.45 | 11.4 | 11.4 | 11.4 | ** |
| 20181020 | Montipora | 3753 | 128 | hard | 12.3 | 12.1 | 12.2 | 10 | 10 | 10 | ** |
| 20181117 | Pocillopora | 1559 | 129 | soft | 50.6 | 48.4 | 49.5 | 43.2 | 43 | 43.1 | 8.4 |
| 20181117 | Pocillopora | 1559 | 130 | hard | 35.6 | 35.2 | 35.4 | 31.6 | 31.2 | 31.4 | 7.8 |
| 20180922 | Pocillopora | 1060 | 131 | soft | 72.2 | 71.4 | 71.8 | 82 | 81.4 | 81.7 | 7.9 |
| 20180922 | Pocillopora | 1060 | 132 | hard | 44.6 | 44 | 44.3 | 50.2 | 50 | 50.1 | 7.7 |
| 20181117 | Montipora | 1478 | 133 | soft | 47.8 | 47.2 | 47.5 | 38.4 | 38.2 | 38.3 | 8 |
| 20181117 | Montipora | 1478 | 134 | hard | 36.2 | 35.8 | 36 | 24.2 | 24 | 24.1 | 8.1 |
| 20181103 | Montipora | 1331 | 135 | soft | 16 | 15.8 | 15.9 | 11.8 | 11.8 | 11.8 | 8.7 |
| 20181103 | Montipora | 1331 | 136 | hard | 10.9 | 10.7 | 10.8 | ** | ** | ** | ** |
| 20181103 | Montipora | 1306 | 137 | soft | 37.6 | 37 | 37.3 | 26.6 | 26.6 | 26.6 | 8.4 |
| 20181103 | Montipora | 1306 | 138 | hard | 31.8 | 31.6 | 31.7 | 13.8 | 13.8 | 13.8 | 8.5 |
Link to 20190725 TapeStation report, Extractions #119-138
Extraction #121 was lighter in color before placing in well.
Extraction #137 did not settle well into the gel. Could be leftover ethanol in the sample.
20190726 E.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here. With the following changes:
- The first and second elutions were spun down in separate microcentrifuge tubes to compare the quantity and quality between the first and second elution.
Coral fragments were randomly chosen from different timepoint bags.
Fragment preparation start 9:19 end 9:29
Soft/Hard homogenization start 9:30 end 9:49
RNA DNA Extraction start 9:50 end 11:40
10 μl aliquots of DNA and 5 μl aliquots of RNA.
Qubit start 11:00 end 12:15
Qubit used to check DNA and RNA quantity (ng/μl).
TapeStation used to check RNA quality.
DNA Standard 1: 178.41 ng/μl
DNA Standard 2: 2043.83 ng/μl
RNA Standard 1: 417.05 ng/μl
RNA Standard 2: 11005.81 ng/μl
| Timepoint | Species | Coral ID | Extraction ID | Homogenization | Elution | DNA Reading 1 | DNA Reading 2 | Average DNA ng/μl | RNA Reading 1 | RNA Reading 2 | Average RNA ng/μl | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
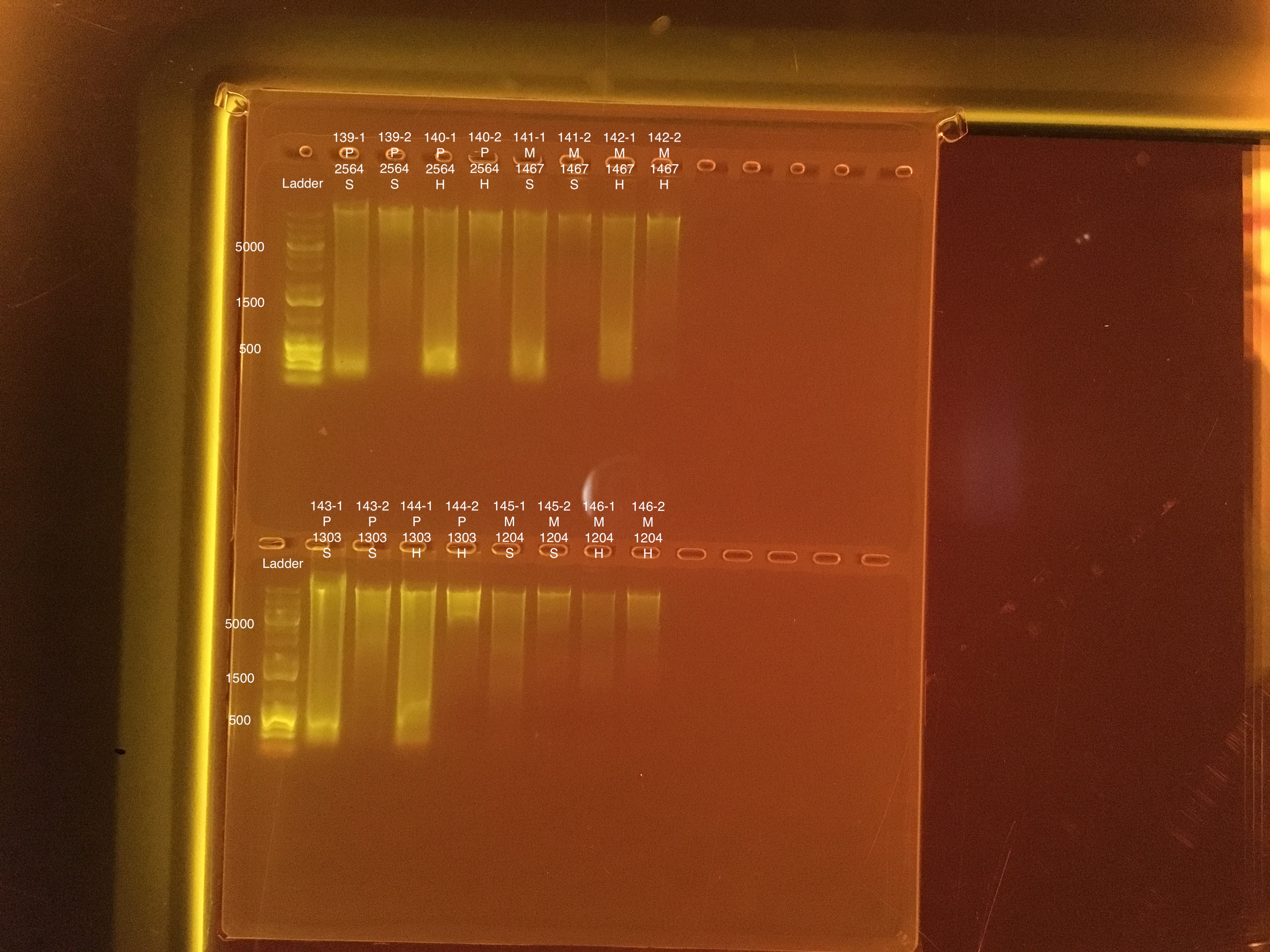
| 20181020 | Pocillopora | 2564 | 139-1 | soft | 1 | 107 | 107 | 107 | 133 | 133 | 133 | 6.7 |
| 20181020 | Pocillopora | 2564 | 139-2 | soft | 2 | 34.2 | 34 | 34.1 | 11.2 | 11.2 | 11.2 | 6.8 |
| 20181020 | Pocillopora | 2564 | 140-1 | hard | 1 | 76.4 | 76 | 76.2 | 88.8 | 89 | 88.9 | 7.5 |
| 20181020 | Pocillopora | 2564 | 140-2 | hard | 2 | 22 | 22 | 22 | 14.8 | 14.8 | 14.8 | 7.8 |
| 20181103 | Montipora | 1467 | 141-1 | soft | 1 | 46.4 | 46.2 | 46.3 | 31.6 | 31.6 | 31.6 | 8.8 |
| 20181103 | Montipora | 1467 | 141-2 | soft | 2 | 9.72 | 9.68 | 9.7 | ** | ** | ** | ** |
| 20181103 | Montipora | 1467 | 142-1 | hard | 1 | 31.8 | 31.6 | 31.7 | 16.2 | 16.2 | 16.2 | 9.2 |
| 20181103 | Montipora | 1467 | 142-2 | hard | 2 | 9.9 | 9.74 | 9.82 | ** | ** | ** | ** |
| 20180923 | Pocillopora | 1303 | 143-1 | soft | 1 | 195 | 194 | 194.5 | 206 | 206 | 206 | 7.5 |
| 20180923 | Pocillopora | 1303 | 143-2 | soft | 2 | 33.6 | 33.6 | 33.6 | 29.2 | 29.2 | 29.2 | 7.1 |
| 20180923 | Pocillopora | 1303 | 144-1 | hard | 1 | 98.4 | 98 | 98.2 | 113 | 113 | 113 | 7.7 |
| 20180923 | Pocillopora | 1303 | 144-2 | hard | 2 | 18.9 | 18.8 | 18.85 | 18.4 | 18.4 | 18.4 | 6.9 |
| 20181117 | Montipora | 1204 | 145-1 | soft | 1 | 13.8 | 13.7 | 13.75 | 21.5 | 21.6 | 21.55 | 9 |
| 20181117 | Montipora | 1204 | 145-2 | soft | 2 | 11.6 | 11.6 | 11.6 | ** | ** | ** | ** |
| 20181117 | Montipora | 1204 | 146-1 | hard | 1 | 6 | 5.96 | 5.98 | 14.4 | 14.4 | 14.4 | 9.4 |
| 20181117 | Montipora | 1204 | 146-2 | hard | 2 | 8.14 | 8.06 | 8.1 | ** | ** | ** | ** |
Link to 20190726 TapeStation report, Extractions #139-146
20190729 E.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here. With the following changes:
- DNA: The first and second elutions were spun down in separate microcentrifuge tubes to compare the quantity and quality between the first and second elution. The first elution had no incubation period (previously 5 minutes) and the second elution had a 15 minute incubation period (previously 5 minutes).
- 10 μl aliquots of DNA and 5 μl aliquots of RNA (instead of 10 μl of each).
Coral fragments were randomly chosen from different timepoint bags.
Fragment preparation start 13:20 end 13:34
Soft/Hard homogenization start 13:35 end 13:55
RNA DNA Extraction start 13:56 end 16:02
Qubit used to check DNA and RNA quantity (ng/μl).
TapeStation used to check RNA quality.
DNA Standard 1: 201.29 ng/μl
DNA Standard 2: 25478.28 ng/μl
RNA Standard 1: 429.76 ng/μl
RNA Standard 2: 11462.14 ng/μl
| Timepoint | Species | Coral ID | Extraction ID | Homogenization | DNA Elution | DNA Reading 1 | DNA Reading 2 | Average DNA ng/μl | RNA Reading 1 | RNA Reading 2 | Average RNA ng/μl | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20180923 | Pocillopora | 1296 | 147 | soft | NA | NA | NA | NA | 81.4 | 81.6 | 81.5 | 8 |
| 20180923 | Pocillopora | 1296 | 147-1 | soft | 1 | 151 | 151 | 151 | NA | NA | NA | NA |
| 20180923 | Pocillopora | 1296 | 147-2 | soft | 2 | 29.4 | 29.8 | 29.6 | NA | NA | NA | NA |
| 20180923 | Pocillopora | 1296 | 148 | hard | NA | NA | NA | NA | 50.2 | 50.2 | 50.2 | 7.7 |
| 20180923 | Pocillopora | 1296 | 148-1 | hard | 1 | 79 | 78.2 | 78.6 | NA | NA | NA | NA |
| 20180923 | Pocillopora | 1296 | 148-2 | hard | 2 | 21.4 | 21.4 | 21.4 | NA | NA | NA | NA |
| 20181103 | Montipora | 2995 | 149 | soft | NA | NA | NA | NA | 16.8 | 17 | 16.9 | 8.2 |
| 20181103 | Montipora | 2995 | 149-1 | soft | 1 | 17.8 | 17.8 | 17.8 | NA | NA | NA | NA |
| 20181103 | Montipora | 2995 | 149-2 | soft | 2 | 11.3 | 11.2 | 11.25 | NA | NA | NA | NA |
| 20181103 | Montipora | 2995 | 150 | hard | NA | NA | NA | NA | 10.6 | 10.6 | 10.6 | 8.3 |
| 20181103 | Montipora | 2995 | 150-1 | hard | 1 | 31.6 | 31.4 | 31.5 | NA | NA | NA | NA |
| 20181103 | Montipora | 2995 | 150-2 | hard | 2 | 9 | 8.98 | 8.99 | NA | NA | NA | NA |
| 20181117 | Pocillopora | 2197 | 151 | soft | NA | NA | NA | NA | 70.2 | 70.2 | 70.2 | 8.6 |
| 20181117 | Pocillopora | 2197 | 151-1 | soft | 1 | 140 | 139 | 139.5 | NA | NA | NA | NA |
| 20181117 | Pocillopora | 2197 | 151-2 | soft | 2 | 40.4 | 40.2 | 40.3 | NA | NA | NA | NA |
| 20181117 | Pocillopora | 2197 | 152 | hard | NA | NA | NA | NA | 47.8 | 47.8 | 47.8 | 8.3 |
| 20181117 | Pocillopora | 2197 | 152-1 | hard | 1 | 62.2 | 62 | 62.1 | NA | NA | NA | NA |
| 20181117 | Pocillopora | 2197 | 152-2 | hard | 2 | 25.4 | 25.4 | 25.4 | NA | NA | NA | NA |
| 20181020 | Montipora | 1779 | 153 | soft | NA | NA | NA | NA | 14.6 | 14.6 | 14.6 | 8.7 |
| 20181020 | Montipora | 1779 | 153-1 | soft | 1 | 25.6 | 25.4 | 25.5 | NA | NA | NA | NA |
| 20181020 | Montipora | 1779 | 153-2 | soft | 2 | 17 | 16.9 | 16.95 | NA | NA | NA | NA |
| 20181020 | Montipora | 1779 | 154 | hard | NA | NA | NA | NA | 10 | 10.2 | 10.1 | ** |
| 20181020 | Montipora | 1779 | 154-1 | hard | 1 | 19.4 | 19.4 | 19.4 | NA | NA | NA | NA |
| 20181020 | Montipora | 1779 | 154-2 | hard | 2 | 16.9 | 16.9 | 16.9 | NA | NA | NA | NA |
Link to 20190729 TapeStation report, Extractions #147-154
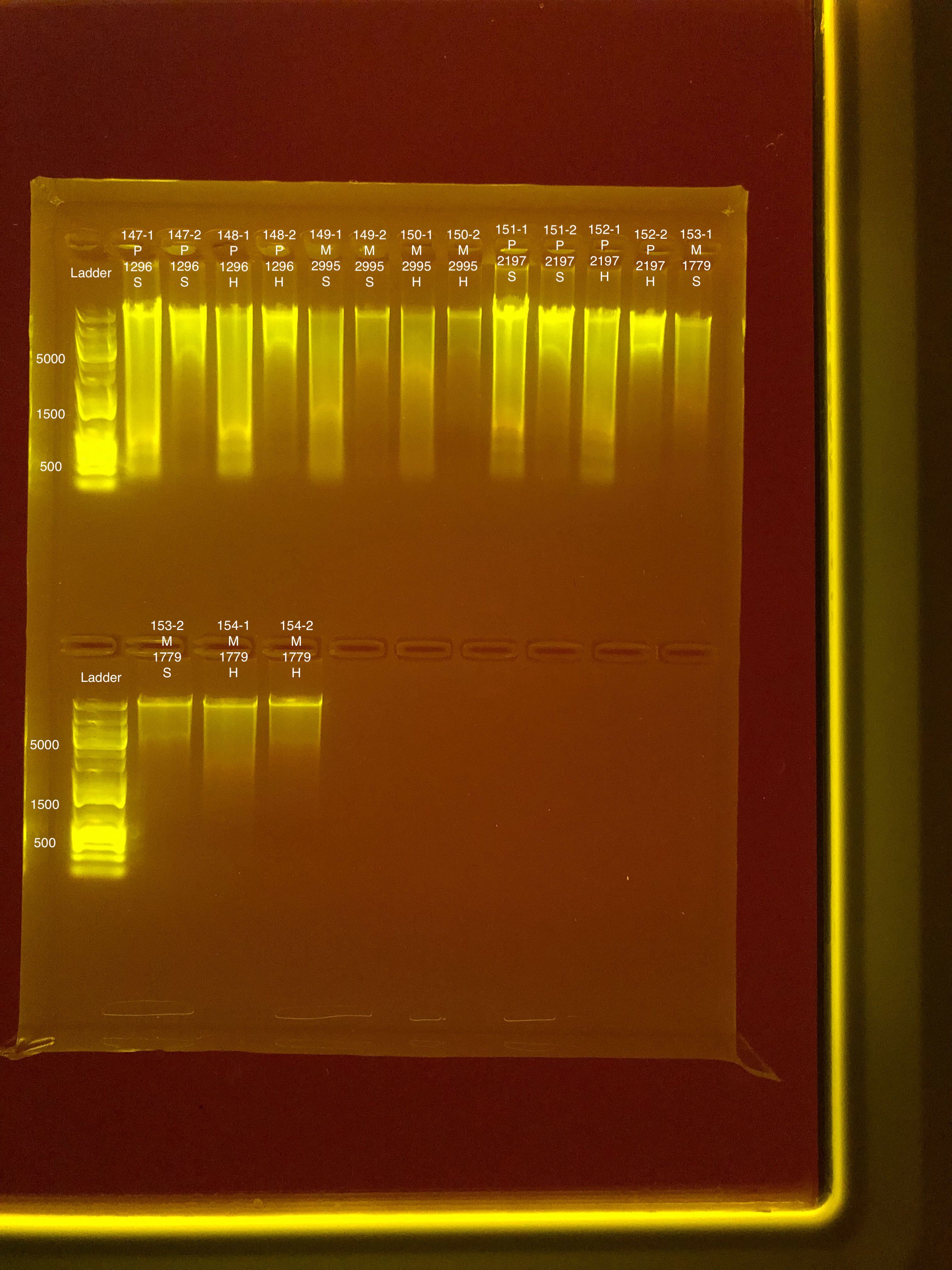
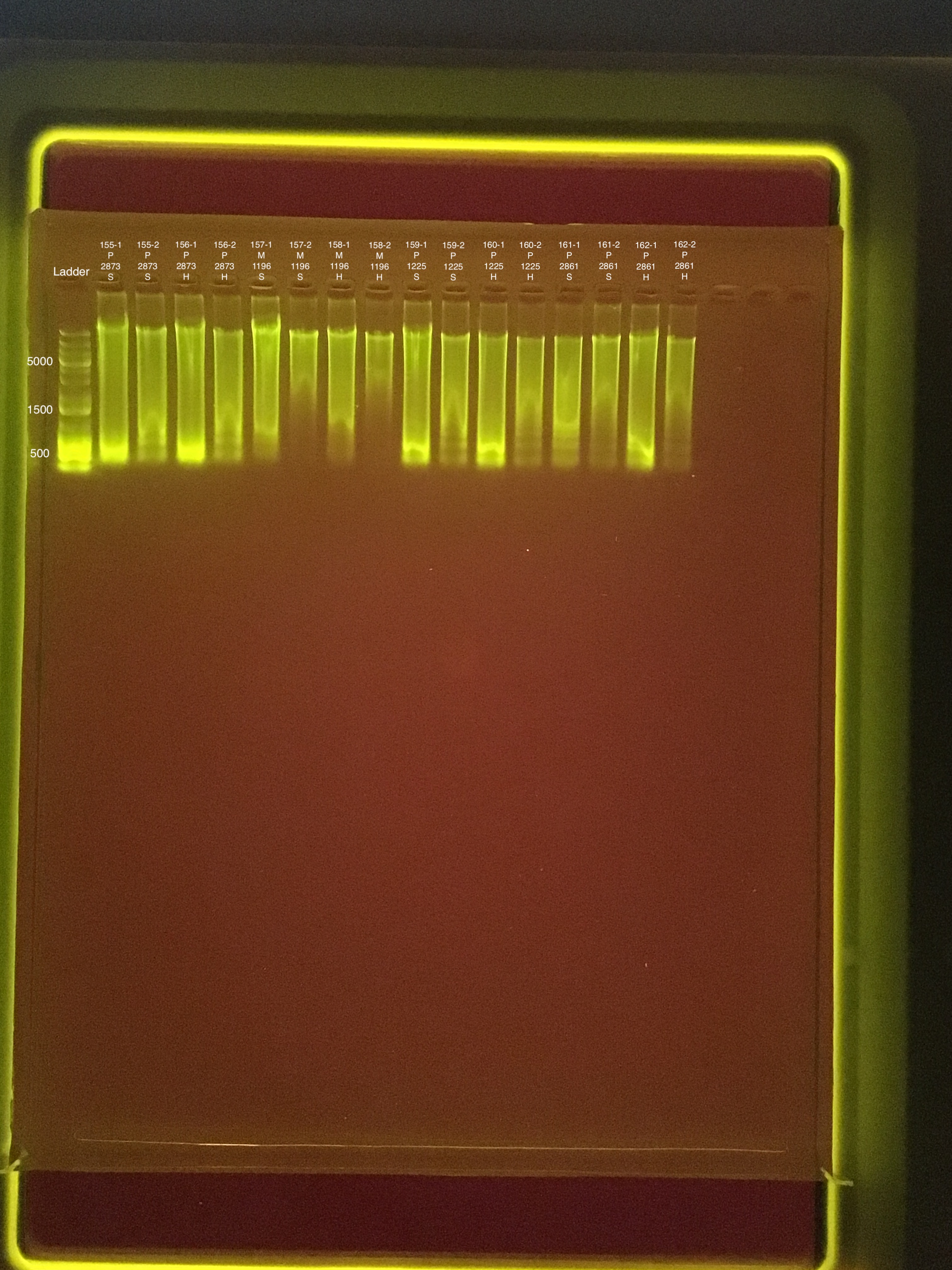
20190730 E.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here. With the following changes:
- DNA: The first and second elutions were spun down in separate microcentrifuge tubes to compare the quantity and quality between the first and second elution. The first elution had no incubation period (previously 5 minutes) with 10 μl of Tris buffer and the second elution had a 15 minute incubation period (previously 5 minutes) with 100 μl of Tris buffer.
- 10 μl aliquots of DNA and 5 μl aliquots of RNA (instead of 10 μl of each).
Coral fragments were randomly chosen from different timepoint bags.
Round 1: Extractions #155-162
Fragment preparation start 8:05 end 8:18
Soft/Hard homogenization start 8:19 end 8:43
RNA DNA Extraction start 8:44 end 10:28
Qubit used to check DNA and RNA quantity (ng/μl).
TapeStation used to check RNA quality.
DNA Standard 1: 211.51 ng/μl
DNA Standard 2: 28121.26 ng/μl
RNA Standard 1: 411.35 ng/μl
RNA Standard 2: 11710.89 ng/μl
| Timepoint | Species | Coral ID | Extraction ID | Homogenization | DNA Elution | DNA Reading 1 | DNA Reading 2 | Average DNA ng/μl | RNA Reading 1 | RNA Reading 2 | Average RNA ng/μl | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20181103 | Pocillopora | 2873 | 155 | soft | NA | NA | NA | NA | 92.8 | 92.6 | 92.7 | ** |
| 20181103 | Pocillopora | 2873 | 155-1 | soft | 1 | 238 | 238 | 238 | NA | NA | NA | NA |
| 20181103 | Pocillopora | 2873 | 155-2 | soft | 2 | 70.2 | 70 | 70.1 | NA | NA | NA | NA |
| 20181103 | Pocillopora | 2873 | 156 | hard | NA | NA | NA | NA | 55 | 54.4 | 54.7 | 7 |
| 20181103 | Pocillopora | 2873 | 156-1 | hard | 1 | 206 | 206 | 206 | NA | NA | NA | NA |
| 20181103 | Pocillopora | 2873 | 156-2 | hard | 2 | 44.2 | 44.2 | 44.2 | NA | NA | NA | NA |
| 20180923 | Montipora | 1196 | 157 | soft | NA | NA | NA | NA | 33.4 | 33.2 | 33.3 | 9.1 |
| 20180923 | Montipora | 1196 | 157-1 | soft | 1 | 204 | 204 | 204 | NA | NA | NA | NA |
| 20180923 | Montipora | 1196 | 157-2 | soft | 2 | 25.6 | 25.6 | 25.6 | NA | NA | NA | NA |
| 20180923 | Montipora | 1196 | 158 | hard | NA | NA | NA | NA | 20.6 | 20.4 | 20.5 | 8.6 |
| 20180923 | Montipora | 1196 | 158-1 | hard | 1 | 85.2 | 85 | 85.1 | NA | NA | NA | NA |
| 20180923 | Montipora | 1196 | 158-2 | hard | 2 | 17.6 | 17.6 | 17.6 | NA | NA | NA | NA |
| 20181117 | Pocillopora | 1225 | 159 | soft | NA | NA | NA | NA | 106 | 106 | 106 | 7.2 |
| 20181117 | Pocillopora | 1225 | 159-1 | soft | 1 | 171 | 171 | 171 | NA | NA | NA | NA |
| 20181117 | Pocillopora | 1225 | 159-2 | soft | 2 | 43.4 | 43.2 | 43.3 | NA | NA | NA | NA |
| 20181117 | Pocillopora | 1225 | 160 | hard | NA | NA | NA | NA | 56.8 | 56.2 | 56.5 | 6.4 |
| 20181117 | Pocillopora | 1225 | 160-1 | hard | 1 | 112 | 112 | 112 | NA | NA | NA | NA |
| 20181117 | Pocillopora | 1225 | 160-2 | hard | 2 | 21.6 | 21.6 | 21.6 | NA | NA | NA | NA |
| 20181020 | Pocillopora | 2861 | 161 | soft | NA | NA | NA | NA | 82.4 | 82.2 | 82.3 | 7.8 |
| 20181020 | Pocillopora | 2861 | 161-1 | soft | 1 | 274 | 274 | 274 | NA | NA | NA | NA |
| 20181020 | Pocillopora | 2861 | 161-2 | soft | 2 | 34.4 | 34.4 | 34.4 | NA | NA | NA | NA |
| 20181020 | Pocillopora | 2861 | 162 | hard | NA | NA | NA | NA | 56.4 | 56 | 56.2 | 7.7 |
| 20181020 | Pocillopora | 2861 | 162-1 | hard | 1 | 139 | 138 | 138.5 | NA | NA | NA | NA |
| 20181020 | Pocillopora | 2861 | 162-2 | hard | 2 | 25.2 | 25.2 | 25.2 | NA | NA | NA | NA |
Link to 20190730 TapeStation report, Extractions #155-162
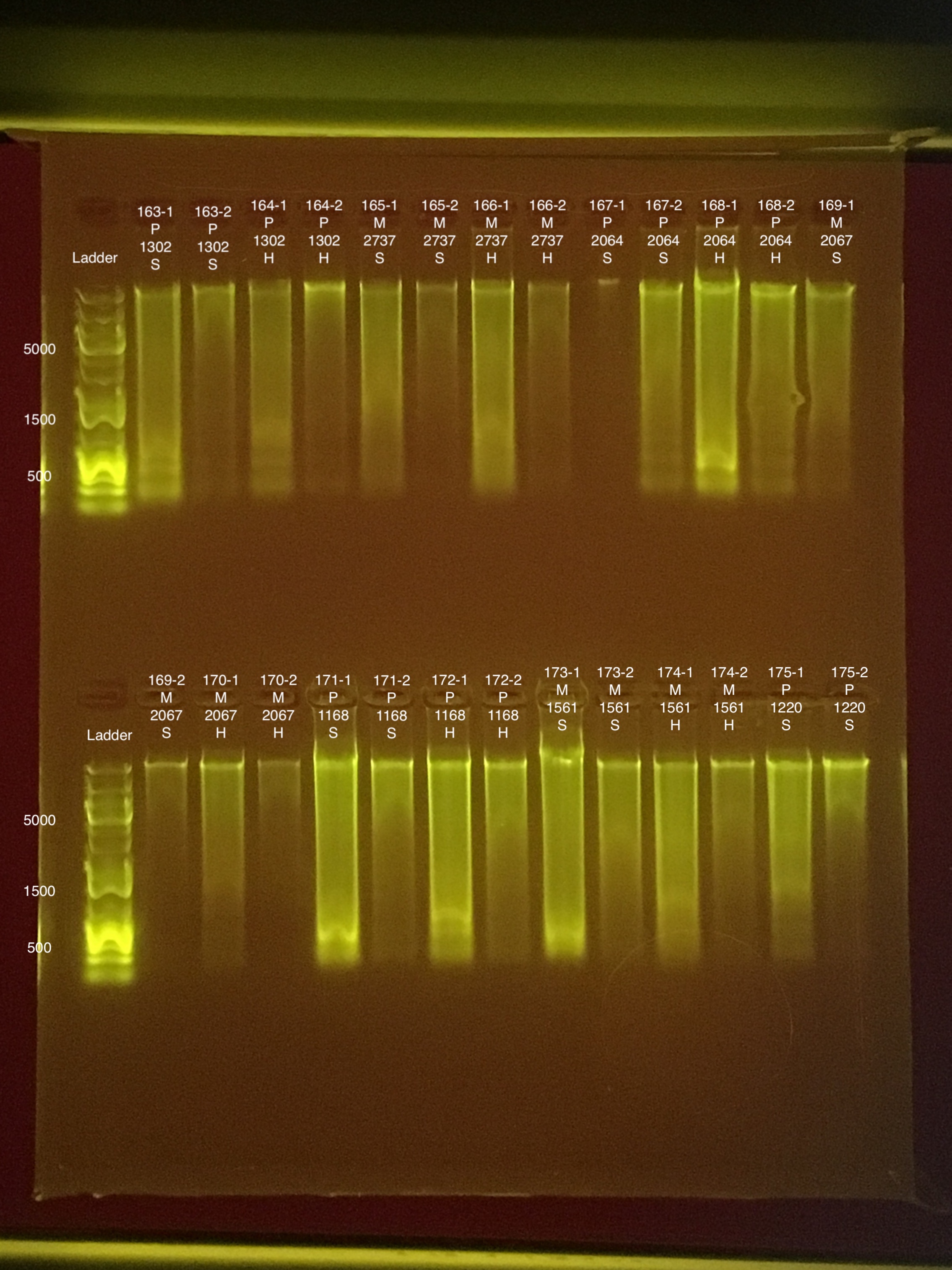
Round 2: Extractions #163-178
Fragment preparation start 13:09 end 13:34
Soft/Hard homogenization start 13:35 end 14:05
RNA DNA Extraction start 14:06 end 16:30
Qubit used to check DNA and RNA quantity (ng/μl).
TapeStation used to check RNA quality.
DNA Standard 1: 209.39 ng/μl
DNA Standard 2: 23646.44 ng/μl
RNA Standard 1: 418.45 ng/μl
RNA Standard 2: 11351.13 ng/μl
| Timepoint | Species | Coral ID | Extraction ID | Homogenization | DNA Elution | DNA Reading 1 | DNA Reading 2 | Average DNA ng/μl | RNA Reading 1 | RNA Reading 2 | Average RNA ng/μl | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20181103 | Pocillopora | 1302 | 163 | soft | NA | NA | NA | NA | 72.8 | 71.6 | 72.2 | 6.8 |
| 20181103 | Pocillopora | 1302 | 163-1 | soft | 1 | 60.8 | 60.2 | 60.5 | NA | NA | NA | NA |
| 20181103 | Pocillopora | 1302 | 163-2 | soft | 2 | 9.4 | 9.26 | 9.33 | NA | NA | NA | NA |
| 20181103 | Pocillopora | 1302 | 164 | hard | NA | NA | NA | NA | 56 | 55.6 | 55.8 | 7.4 |
| 20181103 | Pocillopora | 1302 | 164-1 | hard | 1 | 51.2 | 49.8 | 50.5 | NA | NA | NA | NA |
| 20181103 | Pocillopora | 1302 | 164-2 | hard | 2 | 10.4 | 10.2 | 10.3 | NA | NA | NA | NA |
| 20181117 | Montipora | 2737 | 165 | soft | NA | NA | NA | NA | 21.4 | 21.2 | 21.3 | 9.2 |
| 20181117 | Montipora | 2737 | 165-1 | soft | 1 | 41.2 | 40.8 | 41 | NA | NA | NA | NA |
| 20181117 | Montipora | 2737 | 165-2 | soft | 2 | 6.64 | 6.54 | 6.59 | NA | NA | NA | NA |
| 20181117 | Montipora | 2737 | 166 | hard | NA | NA | NA | NA | 16.2 | 16 | 16.1 | 9.1 |
| 20181117 | Montipora | 2737 | 166-1 | hard | 1 | 92 | 91.6 | 91.8 | NA | NA | NA | NA |
| 20181117 | Montipora | 2737 | 166-2 | hard | 2 | 6.84 | 6.76 | 6.8 | NA | NA | NA | NA |
| 20181117 | Pocillopora | 2064 | 167 | soft | NA | NA | NA | NA | 69.6 | 69.6 | 69.6 | 8.1 |
| 20181117 | Pocillopora | 2064 | 167-1 | soft | 1 | 284 | 282 | 283 | NA | NA | NA | NA |
| 20181117 | Pocillopora | 2064 | 167-2 | soft | 2 | 29 | 28.8 | 28.9 | NA | NA | NA | NA |
| 20181117 | Pocillopora | 2064 | 168 | hard | NA | NA | NA | NA | 63 | 62.4 | 62.7 | 8 |
| 20181117 | Pocillopora | 2064 | 168-1 | hard | 1 | 208 | 206 | 207 | NA | NA | NA | NA |
| 20181117 | Pocillopora | 2064 | 168-2 | hard | 2 | 28.8 | 30.2 | 29.5 | NA | NA | NA | NA |
| 20181020 | Montipora | 2067 | 169 | soft | NA | NA | NA | NA | ** | ** | ** | ** |
| 20181020 | Montipora | 2067 | 169-1 | soft | 1 | 54 | 53.6 | 53.8 | NA | NA | NA | NA |
| 20181020 | Montipora | 2067 | 169-2 | soft | 2 | 6.76 | 6.7 | 6.73 | NA | NA | NA | NA |
| 20181020 | Montipora | 2067 | 170 | hard | NA | NA | NA | NA | ** | ** | ** | 7.7 |
| 20181020 | Montipora | 2067 | 170-1 | hard | 1 | 44 | 43.8 | 43.9 | NA | NA | NA | NA |
| 20181020 | Montipora | 2067 | 170-2 | hard | 2 | 4.38 | 4.34 | 4.36 | NA | NA | NA | NA |
| 20180923 | Pocillopora | 1168 | 171 | soft | NA | NA | NA | NA | 70.8 | 70 | 70.4 | 8.8 |
| 20180923 | Pocillopora | 1168 | 171-2 | soft | 1 | 232 | 232 | 232 | NA | NA | NA | NA |
| 20180923 | Pocillopora | 1168 | 171-2 | soft | 2 | 16 | 15.9 | 15.95 | NA | NA | NA | NA |
| 20180923 | Pocillopora | 1168 | 172 | hard | NA | NA | NA | NA | 60.4 | 59.8 | 60.1 | 8.8 |
| 20180923 | Pocillopora | 1168 | 172-1 | hard | 1 | 125 | 125 | 125 | NA | NA | NA | NA |
| 20180923 | Pocillopora | 1168 | 172-2 | hard | 2 | 21.6 | 21.4 | 21.5 | NA | NA | NA | NA |
| 20181117 | Montipora | 1561 | 173 | soft | NA | NA | NA | NA | 31.2 | 30.2 | 30.7 | 8.6 |
| 20181117 | Montipora | 1561 | 173-1 | soft | 1 | 398 | 396 | 397 | NA | NA | NA | NA |
| 20181117 | Montipora | 1561 | 173-2 | soft | 2 | 22.2 | 22.2 | 22.2 | NA | NA | NA | NA |
| 20181117 | Montipora | 1561 | 174 | hard | NA | NA | NA | NA | 17.6 | 17.4 | 17.5 | 8.8 |
| 20181117 | Montipora | 1561 | 174-1 | hard | 1 | 64.2 | 63.8 | 64 | NA | NA | NA | NA |
| 20181117 | Montipora | 1561 | 174-2 | hard | 2 | 16 | 15.9 | 15.95 | NA | NA | NA | NA |
| 20180922 | Pocillopora | 1220 | 175 | soft | NA | NA | NA | NA | 79.6 | 78.8 | 79.2 | 8.8 |
| 20180922 | Pocillopora | 1220 | 175-1 | soft | 1 | 86.2 | 85.8 | 86 | NA | NA | NA | NA |
| 20180922 | Pocillopora | 1220 | 175-2 | soft | 2 | 24.4 | 24.2 | 24.3 | NA | NA | NA | NA |
| 20180922 | Pocillopora | 1220 | 176 | hard | NA | NA | NA | NA | 57.2 | 56.8 | 57 | 7.8 |
| 20180922 | Pocillopora | 1220 | 176-1 | hard | 1 | 109 | 109 | 109 | NA | NA | NA | NA |
| 20180922 | Pocillopora | 1220 | 176-2 | hard | 2 | 20.4 | 20.2 | 20.3 | NA | NA | NA | NA |
| 20180922 | Montipora | 2000 | 177 | soft | NA | NA | NA | NA | 28.4 | 28 | 28.2 | ** |
| 20180922 | Montipora | 2000 | 177-1 | soft | 1 | 124 | 122 | 123 | NA | NA | NA | NA |
| 20180922 | Montipora | 2000 | 177-2 | soft | 2 | 12.2 | 12.1 | 12.15 | NA | NA | NA | NA |
| 20180922 | Montipora | 2000 | 178 | hard | NA | NA | NA | NA | 19.4 | 19 | 19.2 | 7.4 |
| 20180922 | Montipora | 2000 | 178-1 | hard | 1 | 128 | 128 | 128 | NA | NA | NA | NA |
| 20180922 | Montipora | 2000 | 178-2 | hard | 2 | 19.2 | 18.9 | 19.05 | NA | NA | NA | NA |
Link to 20190730 TapeStation report, Extractions #163-178
20190731 E.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here. With the following changes:
- DNA: The first and second elutions were spun down in separate microcentrifuge tubes to compare the quantity and quality between the first and second elution. The first elution had no incubation period (previously 5 minutes) with 10 μl of Tris buffer and the second elution had a 15 minute incubation period (previously 5 minutes) with 100 μl of Tris buffer.
- 10 μl aliquots of DNA and 5 μl aliquots of RNA (instead of 10 μl of each).
Coral fragments were randomly chosen from different timepoint bags.
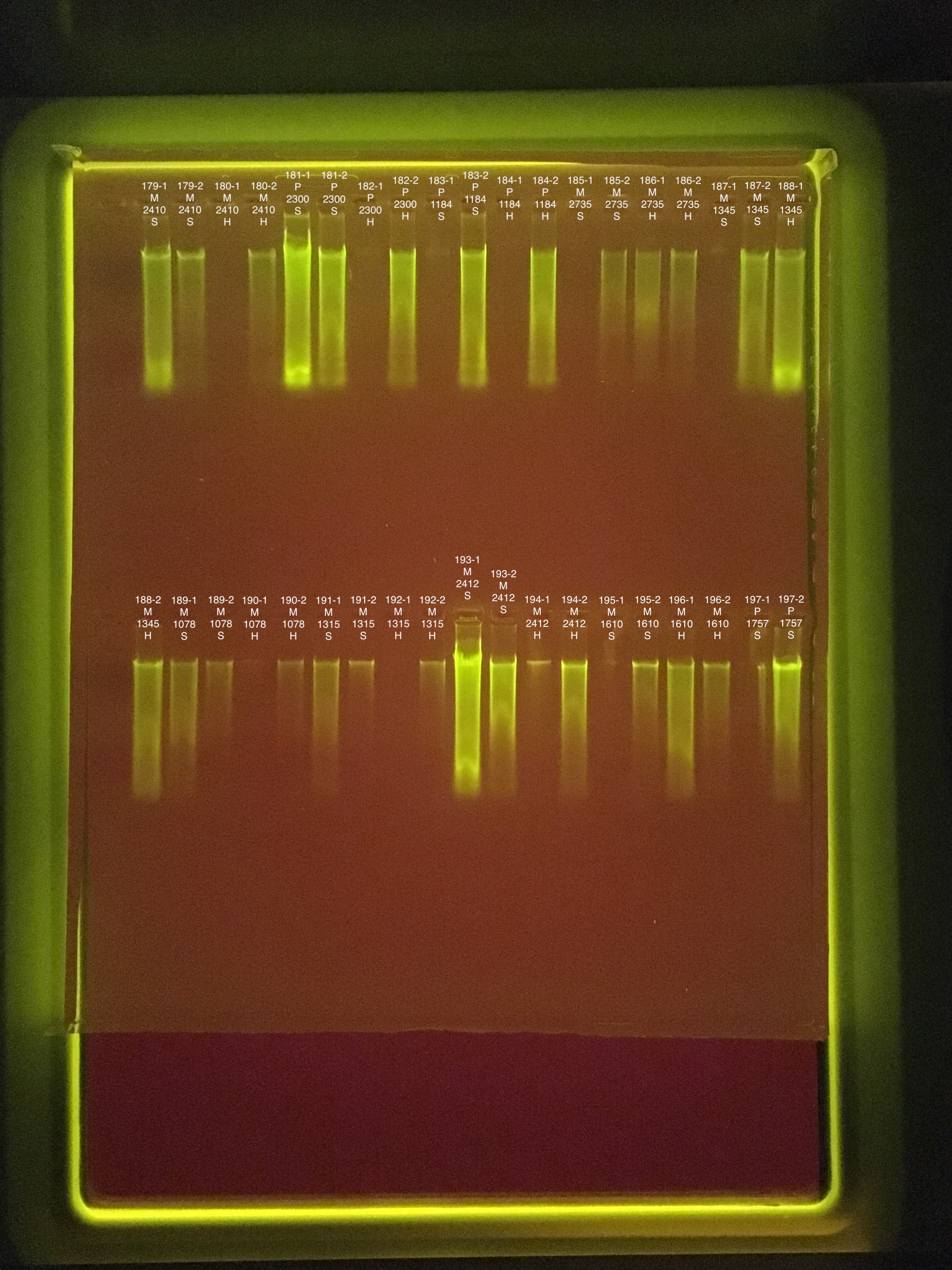
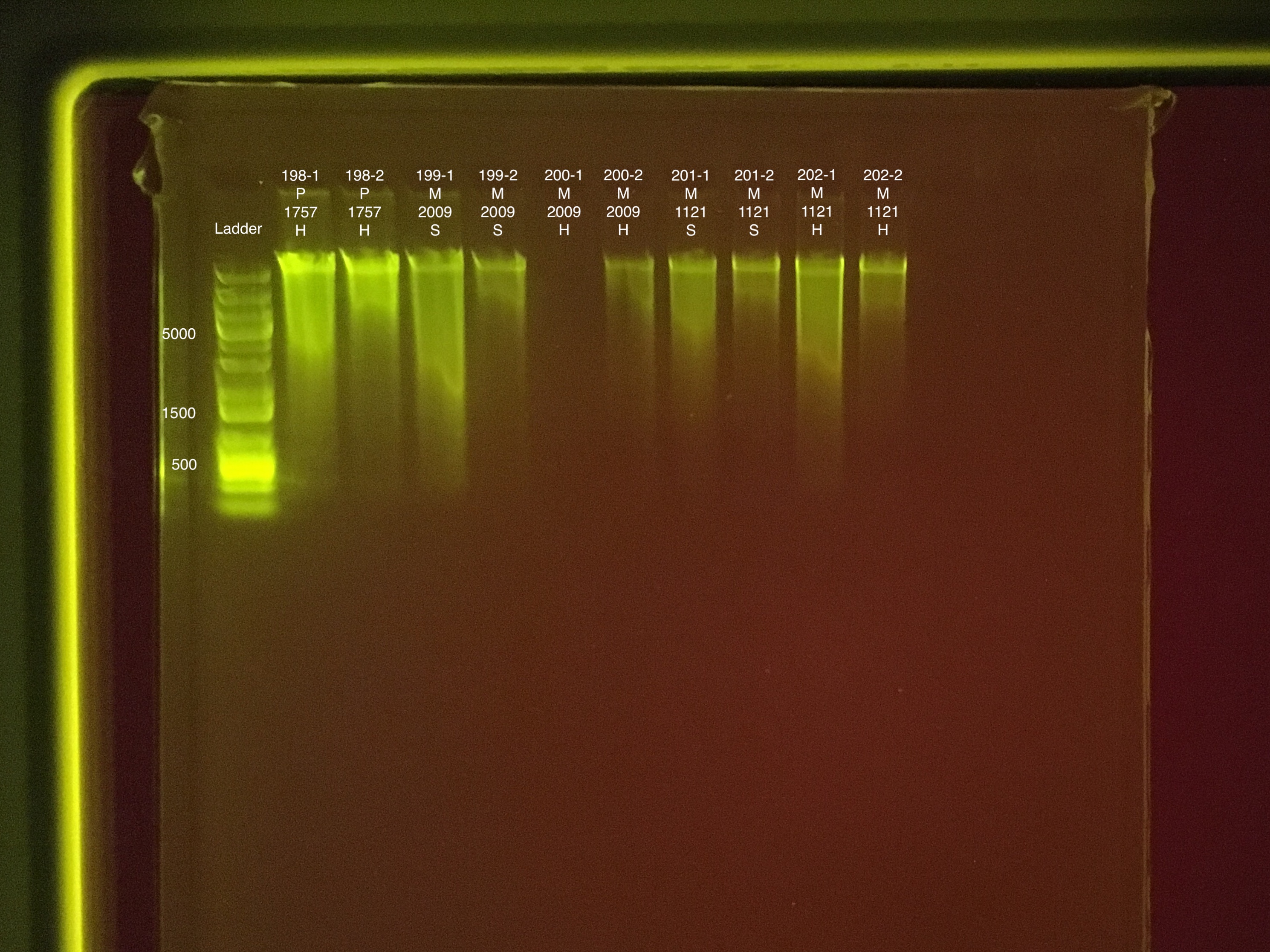
Round 1: Extractions #179-202
Fragment preparation start 8:00 end 8:35
Soft/Hard homogenization start 8:36 end 9:17
RNA DNA Extraction start 9:18 end ~12:30
Qubit used to check DNA and RNA quantity (ng/μl).
TapeStation used to check RNA quality.
DNA Standard 1: 208.49 ng/μl
DNA Standard 2: 24255.12 ng/μl
RNA Standard 1: 393.41 ng/μl
RNA Standard 2: 7871.22 ng/μl
| Timepoint | Species | Coral ID | Extraction ID | Homogenization | DNA Elution | DNA Reading 1 | DNA Reading 2 | Average DNA ng/μl | RNA Reading 1 | RNA Reading 2 | Average RNA ng/μl | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20181020 | Montipora | 2410 | 179 | soft | NA | NA | NA | NA | 16 | 15.8 | 15.9 | ** |
| 20181020 | Montipora | 2410 | 179-1 | soft | 1 | 83.4 | 82.6 | 83 | NA | NA | NA | NA |
| 20181020 | Montipora | 2410 | 179-2 | soft | 2 | 13.2 | 13 | 13.1 | NA | NA | NA | NA |
| 20181020 | Montipora | 2410 | 180 | hard | NA | NA | NA | NA | 12.2 | 12 | 12.1 | ** |
| 20181020 | Montipora | 2410 | 180-1 | hard | 1 | 13 | 12.9 | 12.95 | NA | NA | NA | NA |
| 20181020 | Montipora | 2410 | 180-2 | hard | 2 | 8.7 | 8.6 | 8.65 | NA | NA | NA | NA |
| 20181117 | Pocillopora | 2300 | 181 | soft | NA | NA | NA | NA | 71.2 | 71 | 71.1 | 8.3 |
| 20181117 | Pocillopora | 2300 | 181-1 | soft | 1 | 312 | 308 | 310 | NA | NA | NA | NA |
| 20181117 | Pocillopora | 2300 | 181-2 | soft | 2 | 62.2 | 62 | 62.1 | NA | NA | NA | NA |
| 20181117 | Pocillopora | 2300 | 182 | hard | NA | NA | NA | NA | 60.4 | 59.6 | 60 | 8.6 |
| 20181117 | Pocillopora | 2300 | 182-1 | hard | 1 | 120 | 119 | 119.5 | NA | NA | NA | NA |
| 20181117 | Pocillopora | 2300 | 182-2 | hard | 2 | 33.4 | 33.2 | 33.3 | NA | NA | NA | NA |
| 20181020 | Pocillopora | 1184 | 183 | soft | NA | NA | NA | NA | 85 | 84.8 | 84.9 | 8 |
| 20181020 | Pocillopora | 1184 | 183-1 | soft | 1 | 199 | 198 | 198.5 | NA | NA | NA | NA |
| 20181020 | Pocillopora | 1184 | 183-2 | soft | 2 | 49.2 | 48.8 | 49 | NA | NA | NA | NA |
| 20181020 | Pocillopora | 1184 | 184 | hard | NA | NA | NA | NA | 59.8 | 59.8 | 59.8 | 8.1 |
| 20181020 | Pocillopora | 1184 | 184-1 | hard | 1 | 50.2 | 49.8 | 50 | NA | NA | NA | NA |
| 20181020 | Pocillopora | 1184 | 184-2 | hard | 2 | 34.2 | 33.2 | 33.7 | NA | NA | NA | NA |
| 20181020 | Montipora | 2735 | 185 | soft | NA | NA | NA | NA | 18.6 | 18.8 | 18.7 | ** |
| 20181020 | Montipora | 2735 | 185-1 | soft | 1 | 20.8 | 20.6 | 20.7 | NA | NA | NA | NA |
| 20181020 | Montipora | 2735 | 185-2 | soft | 2 | 6.8 | 6.68 | 6.74 | NA | NA | NA | NA |
| 20181020 | Montipora | 2735 | 186 | hard | NA | NA | NA | NA | 16.4 | 16.2 | 16.3 | ** |
| 20181020 | Montipora | 2735 | 186-1 | hard | 1 | 18.3 | 18.2 | 18.25 | NA | NA | NA | NA |
| 20181020 | Montipora | 2735 | 186-2 | hard | 2 | 8.54 | 8.42 | 8.48 | NA | NA | NA | NA |
| 20180923 | Montipora | 1345 | 187 | soft | NA | NA | NA | NA | 56.2 | 56.2 | 56.2 | 8.4 |
| 20180923 | Montipora | 1345 | 187-1 | soft | 1 | 37.8 | 37.8 | 37.8 | NA | NA | NA | NA |
| 20180923 | Montipora | 1345 | 187-2 | soft | 2 | 25.6 | 25.4 | 25.5 | NA | NA | NA | NA |
| 20180923 | Montipora | 1345 | 188 | hard | NA | NA | NA | NA | 41.8 | 41.8 | 41.8 | 9 |
| 20180923 | Montipora | 1345 | 188-1 | hard | 1 | 169 | 169 | 169 | NA | NA | NA | NA |
| 20180923 | Montipora | 1345 | 188-2 | hard | 2 | 23.6 | 23.4 | 23.5 | NA | NA | NA | NA |
| 20181103 | Montipora | 1078 | 189 | soft | NA | NA | NA | NA | 12.2 | 12 | 12.1 | ** |
| 20181103 | Montipora | 1078 | 189-1 | soft | 1 | 9.32 | 9.2 | 9.26 | NA | NA | NA | NA |
| 20181103 | Montipora | 1078 | 189-2 | soft | 2 | 2.66 | 2.56 | 2.61 | NA | NA | NA | NA |
| 20181103 | Montipora | 1078 | 190 | hard | NA | NA | NA | NA | ** | ** | ** | ** |
| 20181103 | Montipora | 1078 | 190-1 | hard | 1 | 2.76 | 2.82 | 2.79 | NA | NA | NA | NA |
| 20181103 | Montipora | 1078 | 190-2 | hard | 2 | ** | ** | ** | NA | NA | NA | NA |
| 20181117 | Montipora | 1315 | 191 | soft | NA | NA | NA | NA | ** | ** | ** | ** |
| 20181117 | Montipora | 1315 | 191-1 | soft | 1 | 5.44 | 5.38 | 5.41 | NA | NA | NA | NA |
| 20181117 | Montipora | 1315 | 191-2 | soft | 2 | ** | ** | ** | NA | NA | NA | NA |
| 20181117 | Montipora | 1315 | 192 | hard | NA | NA | NA | NA | ** | ** | ** | ** |
| 20181117 | Montipora | 1315 | 192-1 | hard | 1 | ** | ** | ** | NA | NA | NA | NA |
| 20181117 | Montipora | 1315 | 192-2 | hard | 2 | ** | ** | ** | NA | NA | NA | NA |
| 20181103 | Montipora | 2412 | 193 | soft | NA | NA | NA | NA | 45.6 | 45.4 | 45.5 | 8.6 |
| 20181103 | Montipora | 2412 | 193-1 | soft | 1 | 199 | 198 | 198.5 | NA | NA | NA | NA |
| 20181103 | Montipora | 2412 | 193-2 | soft | 2 | 21.6 | 20.8 | 21.2 | NA | NA | NA | NA |
| 20181103 | Montipora | 2412 | 194 | hard | NA | NA | NA | NA | 28.2 | 28.2 | 28.2 | 8.6 |
| 20181103 | Montipora | 2412 | 194-1 | hard | 1 | 68.6 | 68.4 | 68.5 | NA | NA | NA | NA |
| 20181103 | Montipora | 2412 | 194-2 | hard | 2 | 15.2 | 15.1 | 15.15 | NA | NA | NA | NA |
| 20180923 | Montipora | 1610 | 195 | soft | NA | NA | NA | NA | 17.8 | 17.6 | 17.7 | ** |
| 20180923 | Montipora | 1610 | 195-1 | soft | 1 | 11.1 | 11 | 11.05 | NA | NA | NA | NA |
| 20180923 | Montipora | 1610 | 195-2 | soft | 2 | 5.94 | 5.78 | 5.86 | NA | NA | NA | NA |
| 20180923 | Montipora | 1610 | 196 | hard | NA | NA | NA | NA | 19.6 | 19.4 | 19.5 | ** |
| 20180923 | Montipora | 1610 | 196-1 | hard | 1 | 14.5 | 14.4 | 14.45 | NA | NA | NA | NA |
| 20180923 | Montipora | 1610 | 196-2 | hard | 2 | 4.96 | 4.82 | 4.89 | NA | NA | NA | NA |
| 20180923 | Pocillopora | 1757 | 197 | soft | NA | NA | NA | NA | 97 | 96.6 | 96.8 | 8.6 |
| 20180923 | Pocillopora | 1757 | 197-1 | soft | 1 | 77.4 | 77.4 | 77.4 | NA | NA | NA | NA |
| 20180923 | Pocillopora | 1757 | 197-2 | soft | 2 | 48.2 | 47.8 | 48 | NA | NA | NA | NA |
| 20180923 | Pocillopora | 1757 | 198 | hard | NA | NA | NA | NA | 25 | 25 | 25 | 8.3 |
| 20180923 | Pocillopora | 1757 | 198-1 | hard | 1 | 79 | 78.6 | 78.8 | NA | NA | NA | NA |
| 20180923 | Pocillopora | 1757 | 198-2 | hard | 2 | 31.6 | 31.2 | 31.4 | NA | NA | NA | NA |
| 20181103 | Montipora | 2009 | 199 | soft | NA | NA | NA | NA | 20.6 | 20.4 | 20.5 | 5.6 |
| 20181103 | Montipora | 2009 | 199-1 | soft | 1 | 41.2 | 41.4 | 41.3 | NA | NA | NA | NA |
| 20181103 | Montipora | 2009 | 199-2 | soft | 2 | 10.2 | 10.1 | 10.15 | NA | NA | NA | NA |
| 20181103 | Montipora | 2009 | 200 | hard | NA | NA | NA | NA | 11.2 | 11.2 | 11.2 | ** |
| 20181103 | Montipora | 2009 | 200-1 | hard | 1 | 13.5 | 13.5 | 13.5 | NA | NA | NA | NA |
| 20181103 | Montipora | 2009 | 200-2 | hard | 2 | 7.94 | 7.86 | 7.9 | NA | NA | NA | NA |
| 20181103 | Montipora | 1121 | 201 | soft | NA | NA | NA | NA | 20.8 | 20.4 | 20.6 | ** |
| 20181103 | Montipora | 1121 | 201-1 | soft | 1 | 17 | 16.8 | 16.9 | NA | NA | NA | NA |
| 20181103 | Montipora | 1121 | 201-2 | soft | 2 | 6.44 | 6.3 | 6.37 | NA | NA | NA | NA |
| 20181103 | Montipora | 1121 | 202 | hard | NA | NA | NA | NA | 15.6 | 15.4 | 15.5 | ** |
| 20181103 | Montipora | 1121 | 202-1 | hard | 1 | 30 | 30 | 30 | NA | NA | NA | NA |
| 20181103 | Montipora | 1121 | 202-2 | hard | 2 | 5.7 | 5.58 | 5.64 | NA | NA | NA | NA |
| Timepoint | Species | Coral ID | Extraction ID | Homogenization | DNA Elution | DNA Reading 1 | DNA Reading 2 | Average DNA ng/μl | RNA Reading 1 | RNA Reading 2 | Average RNA ng/μl | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 20181020 | Pocillopora | 2012 | 203 | soft | NA | NA | NA | NA | 46.6 | 46.4 | 46.5 | 8.8 |
| 20181020 | Pocillopora | 2012 | 203-1 | soft | 1 | 42 | 40.6 | 41.3 | NA | NA | NA | NA |
| 20181020 | Pocillopora | 2012 | 203-2 | soft | 2 | 19.1 | 18.7 | 18.9 | NA | NA | NA | NA |
| 20181020 | Pocillopora | 2012 | 204 | hard | NA | NA | NA | NA | 60.8 | 60.4 | 60.6 | 8.7 |
| 20181020 | Pocillopora | 2012 | 204-1 | hard | 1 | 242 | 240 | 241 | NA | NA | NA | NA |
| 20181020 | Pocillopora | 2012 | 204-2 | hard | 2 | 36.2 | 36 | 36.1 | NA | NA | NA | NA |
| 20181020 | Montipora | 1436 | 205 | soft | NA | NA | NA | NA | 14.8 | 14.8 | 14.8 | 9.1 |
| 20181020 | Montipora | 1436 | 205-1 | soft | 1 | 91 | 90.6 | 90.8 | NA | NA | NA | NA |
| 20181020 | Montipora | 1436 | 205-2 | soft | 2 | 16.1 | 16.1 | 16.1 | NA | NA | NA | NA |
| 20181020 | Montipora | 1436 | 206 | hard | NA | NA | NA | NA | ** | ** | ** | ** |
| 20181020 | Montipora | 1436 | 206-1 | hard | 1 | 85.8 | 85.6 | 85.7 | NA | NA | NA | NA |
| 20181020 | Montipora | 1436 | 206-2 | hard | 2 | 9.32 | 9.28 | 9.3 | NA | NA | NA | NA |
| 20180929 | Pocillopora | 1254 | 207 | soft | NA | NA | NA | NA | 49.8 | 49.6 | 49.7 | 8 |
| 20180929 | Pocillopora | 1254 | 207-1 | soft | 1 | 280 | 278 | 279 | NA | NA | NA | NA |
| 20180929 | Pocillopora | 1254 | 207-2 | soft | 2 | 43.4 | 43.2 | 43.3 | NA | NA | NA | NA |
| 20180929 | Pocillopora | 1254 | 208 | hard | NA | NA | NA | NA | 32.8 | 32.6 | 32.7 | 8.5 |
| 20180929 | Pocillopora | 1254 | 208-1 | hard | 1 | 169 | 169 | 169 | NA | NA | NA | NA |
| 20180929 | Pocillopora | 1254 | 208-2 | hard | 2 | 32.8 | 32.6 | 32.7 | NA | NA | NA | NA |
| 20181020 | Pocillopora | 1051 | 209 | soft | NA | NA | NA | NA | 75.8 | 75.6 | 75.7 | 8.3 |
| 20181020 | Pocillopora | 1051 | 209-1 | soft | 1 | 248 | 244 | 246 | NA | NA | NA | NA |
| 20181020 | Pocillopora | 1051 | 209-2 | soft | 2 | 86.4 | 86 | 86.2 | NA | NA | NA | NA |
| 20181020 | Pocillopora | 1051 | 210 | hard | NA | NA | NA | NA | 68.6 | 68.6 | 68.6 | 8.2 |
| 20181020 | Pocillopora | 1051 | 210-1 | hard | 1 | 220 | 220 | 220 | NA | NA | NA | NA |
| 20181020 | Pocillopora | 1051 | 210-2 | hard | 2 | 45.6 | 45.4 | 45.5 | NA | NA | NA | NA |
| 20180923 | Montipora | 1776 | 211 | soft | NA | NA | NA | NA | 24.6 | 24.6 | 24.6 | 8.1 |
| 20180923 | Montipora | 1776 | 211-1 | soft | 1 | 50.4 | 50.2 | 50.3 | NA | NA | NA | NA |
| 20180923 | Montipora | 1776 | 211-2 | soft | 2 | 11.5 | 11.4 | 11.45 | NA | NA | NA | NA |
| 20180923 | Montipora | 1776 | 212 | hard | NA | NA | NA | NA | 15.4 | 15.6 | 15.5 | 9 |
| 20180923 | Montipora | 1776 | 212-1 | hard | 1 | 50.8 | 50.4 | 50.6 | NA | NA | NA | NA |
| 20180923 | Montipora | 1776 | 212-2 | hard | 2 | 10.5 | 10.4 | 10.45 | NA | NA | NA | NA |
| 20180923 | Pocillopora | 2877 | 213 | soft | NA | NA | NA | NA | 129 | 129 | 129 | 7.7 |
| 20180923 | Pocillopora | 2877 | 213-1 | soft | 1 | 394 | 394 | 394 | NA | NA | NA | NA |
| 20180923 | Pocillopora | 2877 | 213-2 | soft | 2 | 130 | 129 | 129.5 | NA | NA | NA | NA |
| 20180923 | Pocillopora | 2877 | 214 | hard | NA | NA | NA | NA | 48.2 | 48.2 | 48.2 | 7.7 |
| 20180923 | Pocillopora | 2877 | 214-1 | hard | 1 | 264 | 262 | 263 | NA | NA | NA | NA |
| 20180923 | Pocillopora | 2877 | 214-2 | hard | 2 | 59.2 | 59 | 59.1 | NA | NA | NA | NA |
| 20180929 | Montipora | 1604 | 215 | soft | NA | NA | NA | NA | 15.8 | 15.8 | 15.8 | 8.5 |
| 20180929 | Montipora | 1604 | 215-1 | soft | 1 | 63.6 | 63.4 | 63.5 | NA | NA | NA | NA |
| 20180929 | Montipora | 1604 | 215-2 | soft | 2 | 8.08 | 8 | 8.04 | NA | NA | NA | NA |
| 20180929 | Montipora | 1604 | 216 | hard | NA | NA | NA | NA | 10 | 10 | 10 | ** |
| 20180929 | Montipora | 1604 | 216-1 | hard | 1 | 36 | 35.8 | 35.9 | NA | NA | NA | NA |
| 20180929 | Montipora | 1604 | 216-2 | hard | 2 | 5.8 | 5.66 | 5.73 | NA | NA | NA | NA |
| 20180929 | Montipora | 2555 | 217 | soft | NA | NA | NA | NA | 10.4 | 10.2 | 10.3 | ** |
| 20180929 | Montipora | 2555 | 217-1 | soft | 1 | 23.4 | 23.2 | 23.3 | NA | NA | NA | NA |
| 20180929 | Montipora | 2555 | 217-2 | soft | 2 | 6.26 | 6.2 | 6.23 | NA | NA | NA | NA |
| 20180929 | Montipora | 2555 | 218 | hard | NA | NA | NA | NA | 13.8 | 13.8 | 13.8 | 8.9 |
| 20180929 | Montipora | 2555 | 218-1 | hard | 1 | 30.4 | 30.4 | 30.4 | NA | NA | NA | NA |
| 20180929 | Montipora | 2555 | 218-2 | hard | 2 | 6.66 | 6.6 | 6.63 | NA | NA | NA | NA |
| 20180923 | Montipora | 1583 | 219 | soft | NA | NA | NA | NA | 11.2 | 11.2 | 11.2 | ** |
| 20180923 | Montipora | 1583 | 219-1 | soft | 1 | 49 | 48.8 | 48.9 | NA | NA | NA | NA |
| 20180923 | Montipora | 1583 | 219-2 | soft | 2 | 7.44 | 7.38 | 7.41 | NA | NA | NA | NA |
| 20180923 | Montipora | 1583 | 220 | hard | NA | NA | NA | NA | ** | ** | ** | ** |
| 20180923 | Montipora | 1583 | 220-1 | hard | 1 | 32.6 | 32.4 | 32.5 | NA | NA | NA | NA |
| 20180923 | Montipora | 1583 | 220-2 | hard | 2 | 5.3 | 5.04 | 5.17 | NA | NA | NA | NA |
| 20180929 | Pocillopora | 1466 | 221 | soft | NA | NA | NA | NA | 58.8 | 58.8 | 58.8 | 8.3 |
| 20180929 | Pocillopora | 1466 | 221-1 | soft | 1 | 308 | 306 | 307 | NA | NA | NA | NA |
| 20180929 | Pocillopora | 1466 | 221-2 | soft | 2 | 55 | 54.6 | 54.8 | NA | NA | NA | NA |
| 20180929 | Pocillopora | 1466 | 222 | hard | NA | NA | NA | NA | 61.2 | 61.2 | 61.2 | 7.8 |
| 20180929 | Pocillopora | 1466 | 222-1 | hard | 1 | 196 | 196 | 196 | NA | NA | NA | NA |
| 20180929 | Pocillopora | 1466 | 222-2 | hard | 2 | 32.8 | 32.8 | 32.8 | NA | NA | NA | NA |
| 20180929 | Montipora | 2016 | 223 | soft | NA | NA | NA | NA | 10.8 | 10.8 | 10.8 | ** |
| 20180929 | Montipora | 2016 | 223-1 | soft | 1 | 22 | 21.8 | 21.9 | NA | NA | NA | NA |
| 20180929 | Montipora | 2016 | 223-2 | soft | 2 | 5.2 | 5.14 | 5.17 | NA | NA | NA | NA |
| 20180929 | Montipora | 2016 | 224 | hard | NA | NA | NA | NA | ** | ** | ** | ** |
| 20180929 | Montipora | 2016 | 224-1 | hard | 1 | 26.4 | 26.4 | 26.4 | NA | NA | NA | NA |
| 20180929 | Montipora | 2016 | 224-2 | hard | 2 | 4.36 | 4.32 | 4.34 | NA | NA | NA | NA |
| 20180923 | Montipora | 1321 | 225 | soft | NA | NA | NA | NA | ** | ** | ** | ** |
| 20180923 | Montipora | 1321 | 225-1 | soft | 1 | 45.4 | 45.4 | 45.4 | NA | NA | NA | NA |
| 20180923 | Montipora | 1321 | 225-2 | soft | 2 | 6.42 | 6.38 | 6.4 | NA | NA | NA | NA |
| 20180923 | Montipora | 1321 | 226 | hard | NA | NA | NA | NA | ** | ** | ** | ** |
| 20180923 | Montipora | 1321 | 226-1 | hard | 1 | 28.6 | 28.6 | 28.6 | NA | NA | NA | NA |
| 20180923 | Montipora | 1321 | 226-2 | hard | 2 | 6.7 | 6.62 | 6.66 | NA | NA | NA | NA |
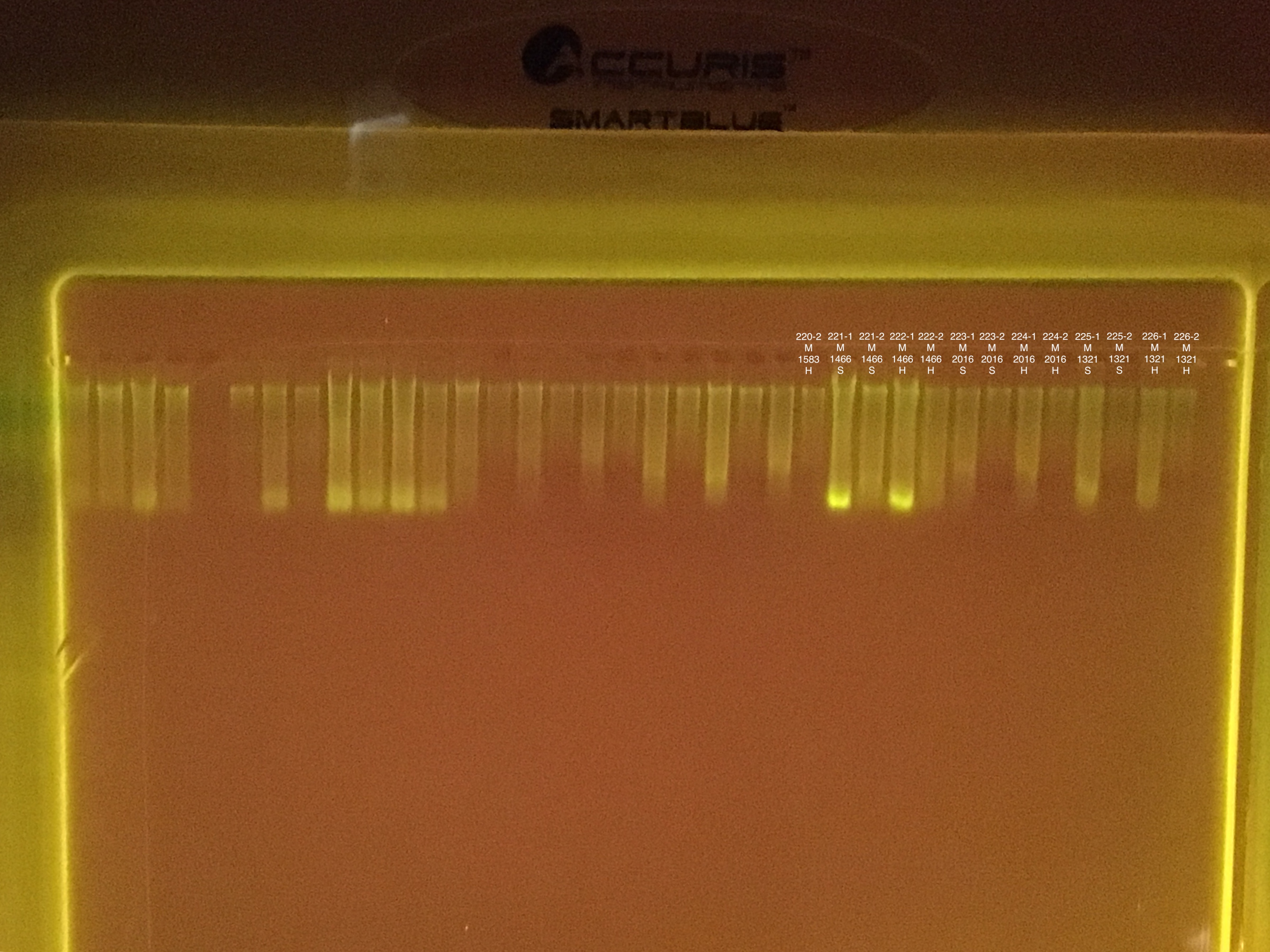
Fragment #2300 is very bleached with algae on the top of some of the branches. Pieces taken from the no algae portions.
Link to 20190731 TapeStation report, Extractions #179-202
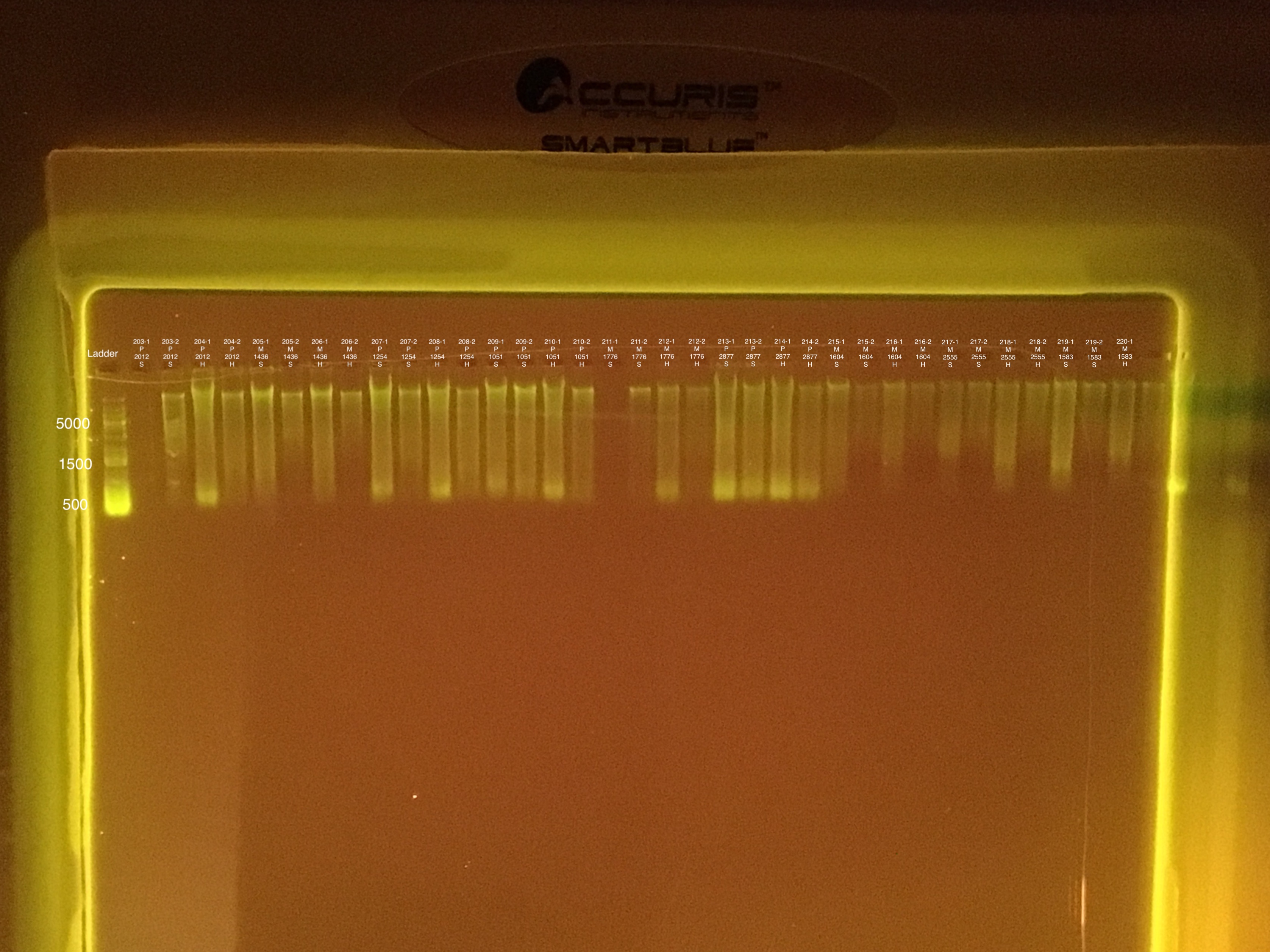
Several samples did not sink into the well. Possibly because of excess ethanol.