Holobiont Integration August DNA RNA Extractions
August DNA RNA Extractions
20190801 E.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here. With the following changes:
- DNA: The first and second elutions were spun down in separate microcentrifuge tubes to compare the quantity and quality between the first and second elution. The first elution had no incubation period (previously 5 minutes) with 10 μl of Tris buffer and the second elution had a 15 minute incubation period (previously 5 minutes) with 100 μl of Tris buffer.
- 10 μl aliquots of DNA and 5 μl aliquots of RNA (instead of 10 μl of each).
Coral fragments were randomly chosen from different timepoint bags.
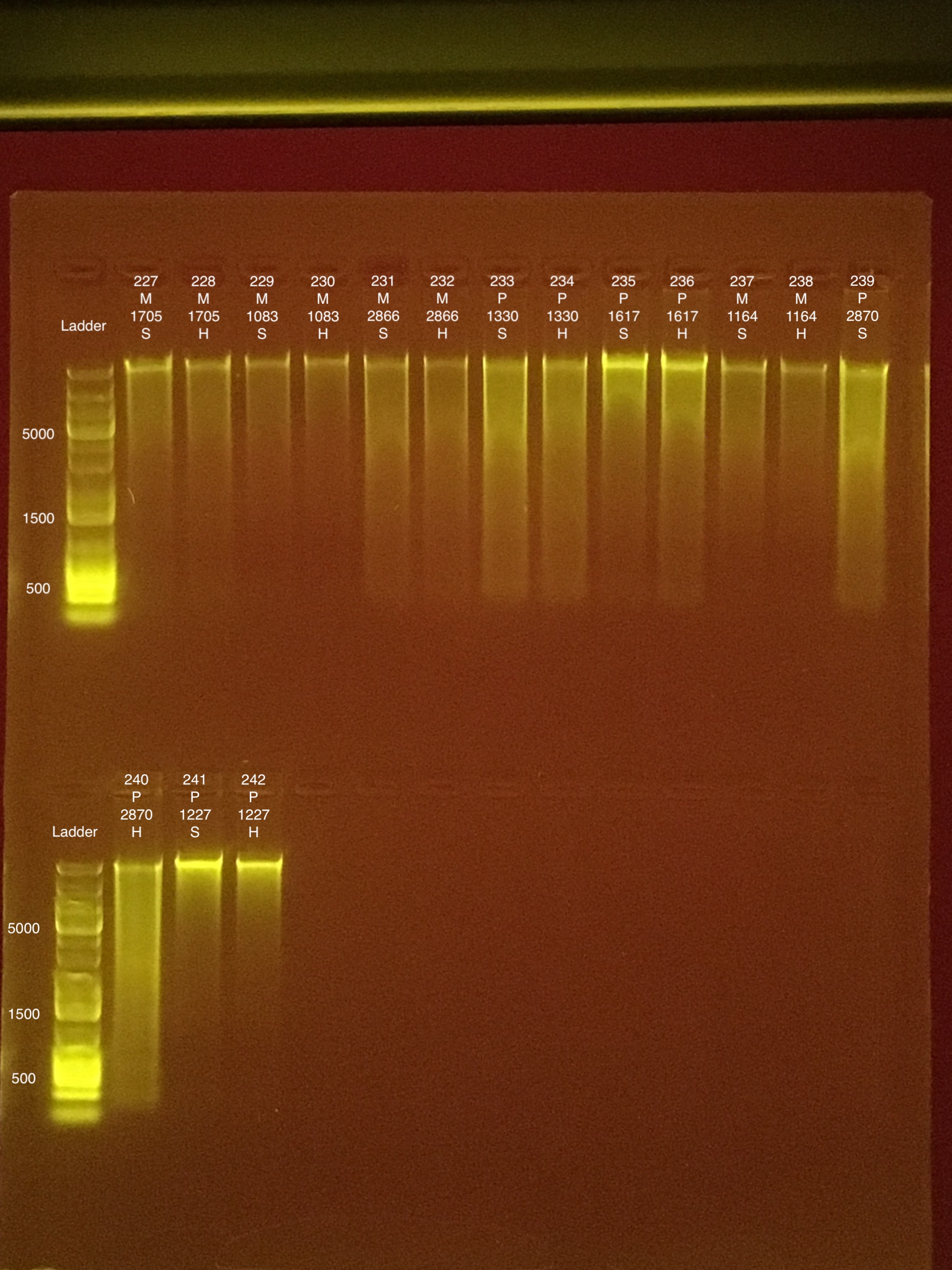
Round 1: Extractions #227-242:
Fragment preparation start 10:00 end 10:15
Soft/Hard homogenization start 10:16 end NA
RNA DNA Extraction start NA end ~13:30
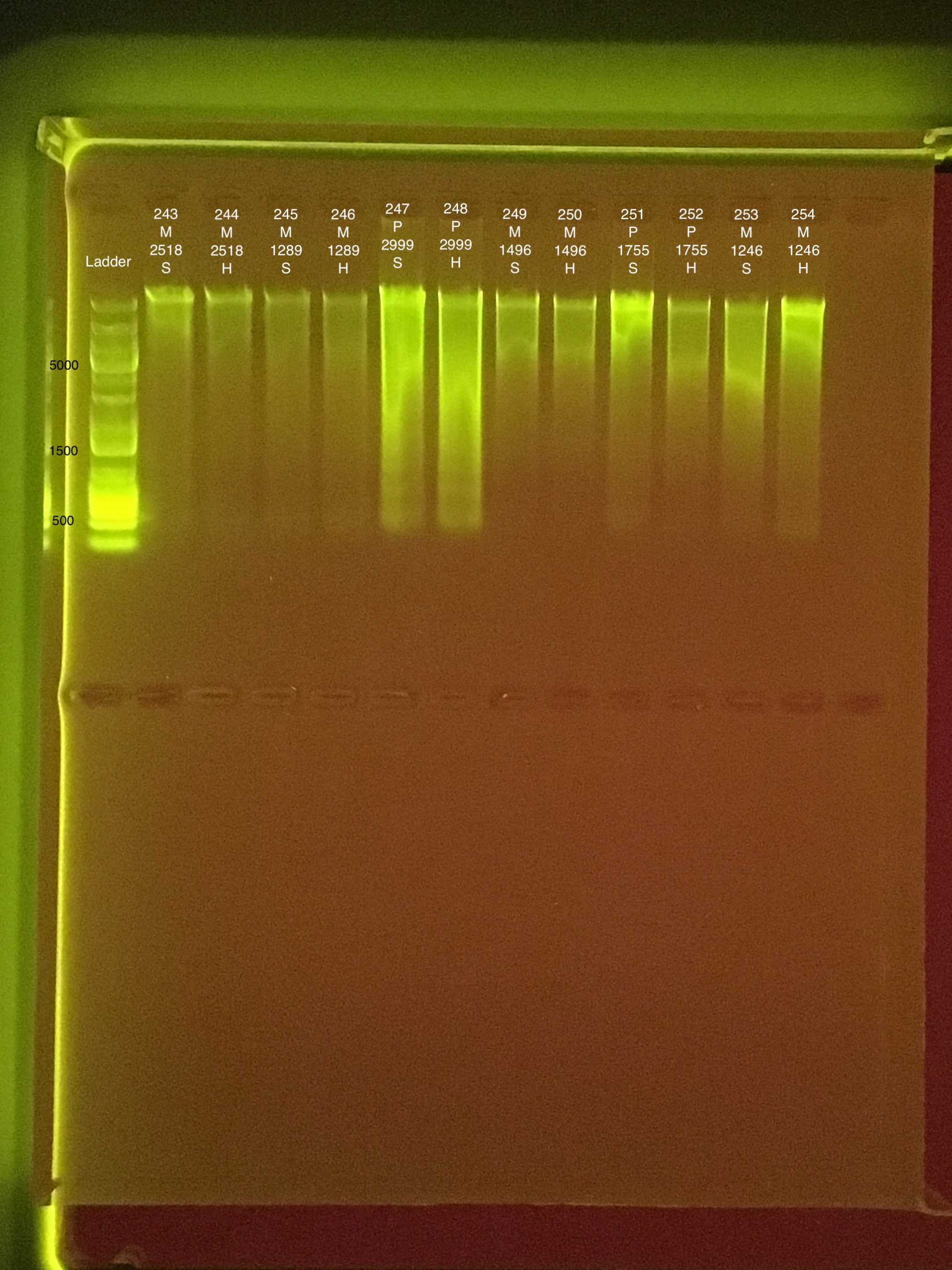
No time data for Round 2: Extractions #243-254
Qubit used to check DNA and RNA quantity (ng/μl).
TapeStation used to check RNA quality.
Elution 2 of the DNA extractions not measured on Qubit.
Round 1: Extractions #227-242:
DNA Standard 1: 196.29 ng/μl
DNA Standard 2: 21,117.67 ng/μl
RNA Standard 1: 399.45 ng/μl
RNA Standard 2: 9,024.70 ng/μl
Round 2: Extractions #243-254:
DNA Standard 1: 191.06 ng/μl
DNA Standard 2: 20,916.33 ng/μl
RNA Standard 1: 392.22 ng/μl
RNA Standard 2: 10,100.11 ng/μl
| Timepoint | Species | Coral ID | Extraction ID | Homogenization | DNA Reading 1 | DNA Reading 2 | Average DNA ng/μl | RNA Reading 1 | RNA Reading 2 | Average RNA ng/μl | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20180929 | Montipora | 1705 | 227 | soft | 16.3 | 16.1 | 16.2 | 60.2 | 59.8 | 60 | 8.7 |
| 20180929 | Montipora | 1705 | 228 | hard | 11.9 | 11.8 | 11.85 | 27.2 | 27.2 | 27.2 | 8.5 |
| 20181020 | Montipora | 1083 | 229 | soft | 8.42 | 8.38 | 8.4 | 22 | 21.8 | 21.9 | 9.4 |
| 20181020 | Montipora | 1083 | 230 | hard | 6.98 | 6.94 | 6.96 | 13.2 | 13.2 | 13.2 | 8.6 |
| 20180922 | Montipora | 2866 | 231 | soft | 14.8 | 14.7 | 14.75 | 46.4 | 46.4 | 46.4 | 8.8 |
| 20180922 | Montipora | 2866 | 232 | hard | 10.8 | 10.8 | 10.8 | 20.4 | 20.4 | 20.4 | 7.5 |
| 20180929 | Pocillopora | 1330 | 233 | soft | 25 | 24.8 | 24.9 | 110 | 110 | 110 | 8.3 |
| 20180929 | Pocillopora | 1330 | 234 | hard | 16.9 | 16.7 | 16.8 | 82.6 | 82.4 | 82.5 | 8.8 |
| 20180922 | Pocillopora | 1617 | 235 | soft | 24 | 24 | 24 | 91.8 | 91.6 | 91.7 | 8.5 |
| 20180922 | Pocillopora | 1617 | 236 | hard | 30 | 29.8 | 29.9 | 114 | 114 | 114 | 7.8 |
| 20180929 | Montipora | 1164 | 237 | soft | 15.2 | 15.1 | 15.15 | 46.4 | 45.8 | 46.1 | 7.9 |
| 20180929 | Montipora | 1164 | 238 | hard | 8.86 | 8.76 | 8.81 | 19.2 | 19 | 19.1 | 9.3 |
| 20180929 | Pocillopora | 2870 | 239 | soft | 39.2 | 39 | 39.1 | 129 | 129 | 129 | 8.9 |
| 20180929 | Pocillopora | 2870 | 240 | hard | 36.2 | 35.8 | 36 | 120 | 120 | 120 | 8.6 |
| 20180922 | Pocillopora | 1227 | 241 | soft | 15.5 | 15.3 | 15.4 | 99.8 | 98.8 | 99.3 | 6.9 |
| 20180922 | Pocillopora | 1227 | 242 | hard | 10.6 | 10.5 | 10.55 | 146 | 144 | 145 | 7.9 |
| 20180922 | Montipora | 2518 | 243 | soft | 14 | 13.8 | 13.9 | 18.4 | 18.2 | 18.3 | 9.2 |
| 20180922 | Montipora | 2518 | 244 | hard | 8.32 | 9.28 | 8.8 | 18 | 17.8 | 17.9 | 8.8 |
| 20180922 | Montipora | 1289 | 245 | soft | 7.44 | 7.4 | 7.42 | 14.6 | 14.2 | 14.4 | ** |
| 20180922 | Montipora | 1289 | 246 | hard | 7.52 | 7.5 | 7.51 | 10.6 | 10.8 | 10.7 | ** |
| 20180929 | Pocillopora | 2999 | 247 | soft | 54.2 | 54.2 | 54.2 | 106 | 105 | 105.5 | 7 |
| 20180929 | Pocillopora | 2999 | 248 | hard | 44 | 44 | 44 | 96 | 95.4 | 95.7 | 7 |
| 20180929 | Montipora | 1496 | 249 | soft | 12.2 | 12.1 | 12.15 | 18.4 | 18.2 | 18.3 | 8.8 |
| 20180929 | Montipora | 1496 | 250 | hard | 9.9 | 9.88 | 9.89 | 11.4 | 11.2 | 11.3 | ** |
| 20181020 | Pocillopora | 1755 | 251 | soft | 19.3 | 19.2 | 19.25 | 57 | 56.4 | 56.7 | 8.2 |
| 20181020 | Pocillopora | 1755 | 252 | hard | 12.4 | 11.6 | 12 | 19.6 | 19.6 | 19.6 | 8.6 |
| 20181020 | Montipora | 1246 | 253 | soft | 19.1 | 18.9 | 19 | 28.4 | 28 | 28.2 | 8.8 |
| 20181020 | Montipora | 1246 | 254 | hard | 19.8 | 19.9 | 19.85 | 53.4 | 52.8 | 53.1 | 8.2 |
Link to 20190801 TapeStation report, Extractions #227-242
Extractions #243-254 ran on 20190802 with extractions #255-266.
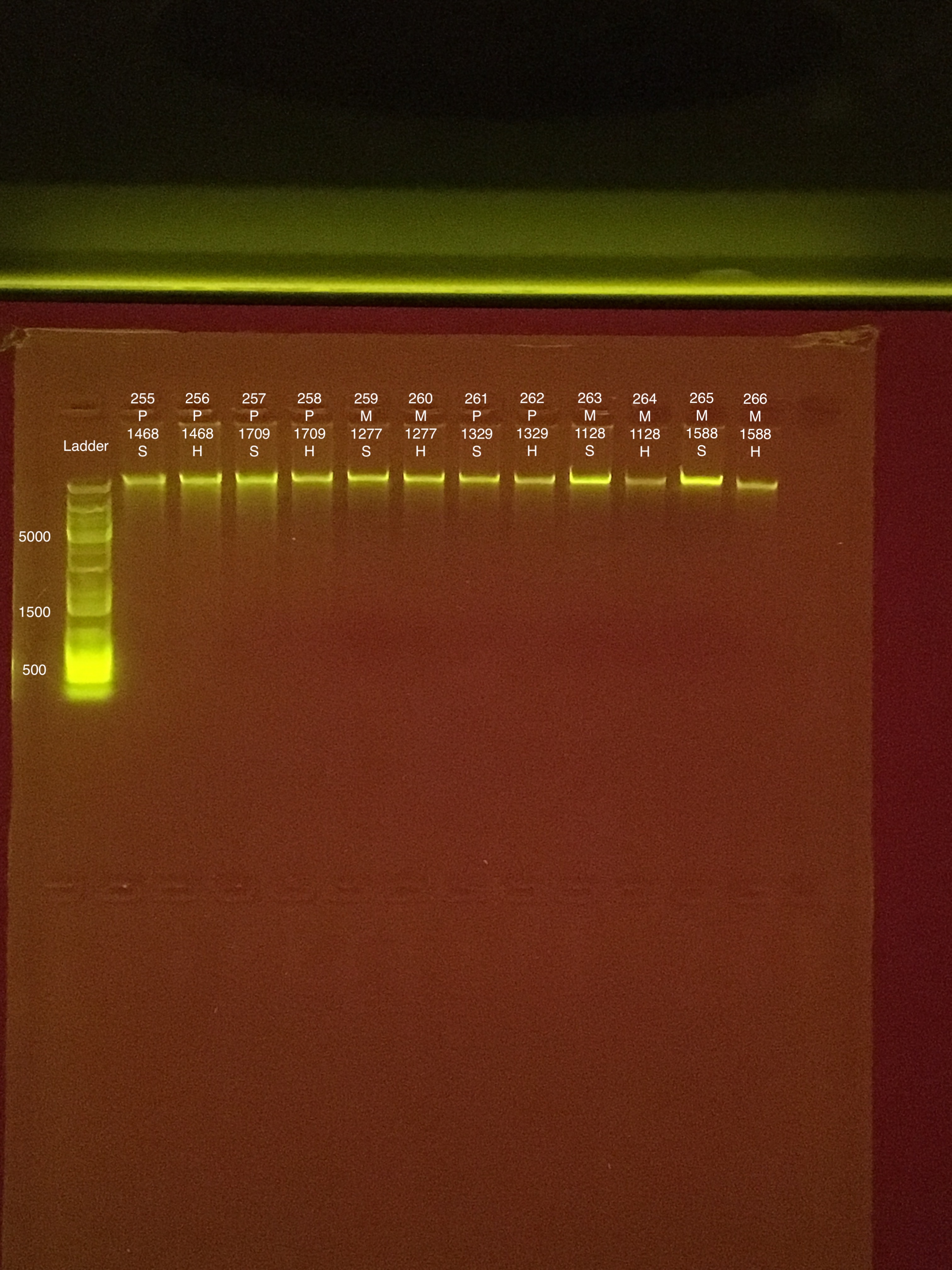
Link to 20190802 TapeStation report, Extractions #255-266
20190802 E.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here. With the following changes:
- DNA: The first and second elutions were spun down in separate microcentrifuge tubes to compare the quantity and quality between the first and second elution. The first elution had no incubation period (previously 5 minutes) with 10 μl of Tris buffer and the second elution had a 15 minute incubation period (previously 5 minutes) with 100 μl of Tris buffer.
- 10 μl aliquots of DNA and 5 μl aliquots of RNA (instead of 10 μl of each).
Coral fragments were randomly chosen from different timepoint bags.
DNA samples atypically put in green spin-away columns (green columns are usually used for RNA samples), but Zymo webpage states green spin-away columns can be used for DNA and/or RNA. DNA samples measured on the Qubit before proceeding with RNA samples. DNA/RNA shield atypically used instead of Wash Buffer during DNA extraction.
DNA samples #260 and #266 have final elution volume of 80 μl.
Fragment preparation start 9:25 end 9:43
Soft/Hard homogenization start 9:44 end 10:10
RNA DNA Extraction start 10:11 end 12:51
Qubit used to check DNA and RNA quantity (ng/μl).
TapeStation used to check RNA quality.
DNA Standard 1: 188.62 ng/μl
DNA Standard 2: 18,548.07 ng/μl
RNA Standard 1: 389.8 ng/μl
RNA Standard 2: 10,185.69 ng/μl
| Timepoint | Species | Coral ID | Extraction ID | Homogenization | DNA Reading 1 | DNA Reading 2 | Average DNA ng/μl | RNA Reading 1 | RNA Reading 2 | Average RNA ng/μl | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20180929 | Pocillopora | 1468 | 255 | soft | 6.32 | 6.16 | 6.24 | 131 | 130 | 130.5 | 6.7 |
| 20180929 | Pocillopora | 1468 | 256 | hard | 10.4 | 10.3 | 10.35 | 78 | 77.8 | 77.9 | 6.5 |
| 20181020 | Pocillopora | 1709 | 257 | soft | 8.48 | 8.46 | 8.47 | 77.4 | 77.2 | 77.3 | 7.7 |
| 20181020 | Pocillopora | 1709 | 258 | hard | 6.82 | 6.78 | 6.8 | 70.2 | 70 | 70.1 | 7 |
| 20180922 | Montipora | 1277 | 259 | soft | 7.68 | 7.62 | 7.65 | 48.6 | 48.6 | 48.6 | 8.9 |
| 20180922 | Montipora | 1277 | 260 | hard | 5.96 | 5.94 | 5.95 | 27.6 | 27.6 | 27.6 | 8.9 |
| 20181020 | Pocillopora | 1329 | 261 | soft | 4.96 | 4.94 | 4.95 | 74.6 | 74.4 | 74.5 | 8.3 |
| 20181020 | Pocillopora | 1329 | 262 | hard | 6.38 | 6.36 | 6.37 | 71 | 71 | 71 | 7.7 |
| 20180922 | Montipora | 1128 | 263 | soft | 10.7 | 10.6 | 10.65 | 47 | 47 | 47 | 8.7 |
| 20180922 | Montipora | 1128 | 264 | hard | 3.74 | 3.74 | 3.74 | 30.2 | 30.4 | 30.3 | 8.9 |
| 20180929 | Montipora | 1588 | 265 | soft | 8.96 | 8.94 | 8.95 | 25.2 | 25.2 | 25.2 | 9.4 |
| 20180929 | Montipora | 1588 | 266 | hard | 4.66 | 4.66 | 4.66 | 20 | 20 | 20 | 9.6 |
Link to 20190802 TapeStation report, Extractions #255-266
20190805 E.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here. With the following changes:
- DNA: The first and second elutions were spun down in separate microcentrifuge tubes to compare the quantity and quality between the first and second elution. The first elution had no incubation period (previously 5 minutes) with 10 μl of Tris buffer and the second elution had a 15 minute incubation period (previously 5 minutes) with 100 μl of Tris buffer.
- 10 μl aliquots of DNA and 5 μl aliquots of RNA (instead of 10 μl of each).
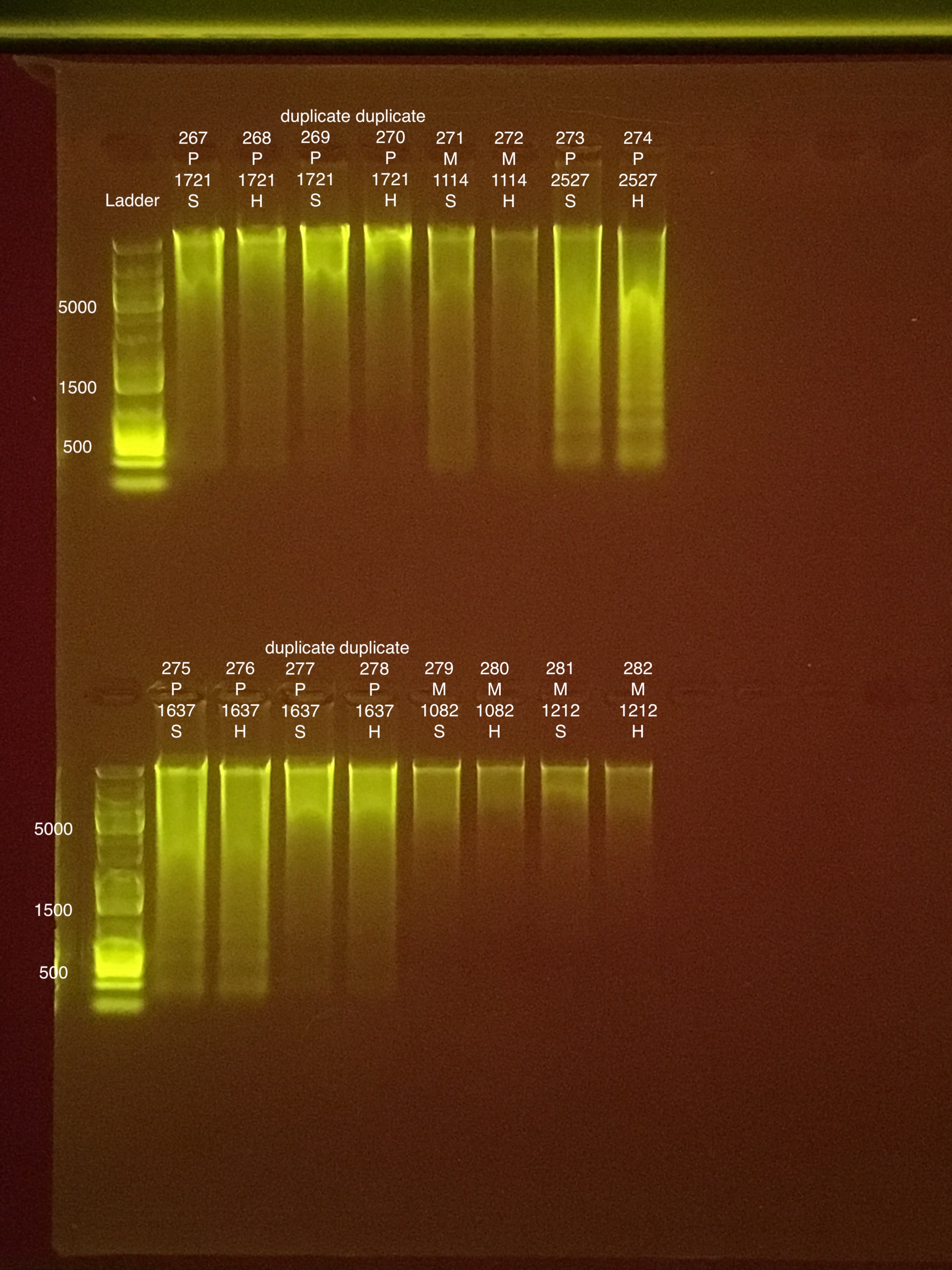
- 2 coral fragments were used twice (6 coral fragments, 2 of which were done twice. 8 bead tubes used total) to compare DNA quality after the addition of a RNA/DNA shield wash. 500 μl of RNA/DNA shield “wash” step added before the 700 μl of Wash Buffer step for the duplicate extractions.
Coral fragments were randomly chosen from different timepoint bags.
Fragment preparation start 11:00 end 11:18
Soft/Hard homogenization start 11:19 end 11:51
RNA DNA Extraction start 11:52 end 14:42
Extraction #s: 269, 270, 277, and 278 got the 500 μl RNA/DNA shield “wash” step in the DNA extractions only.
Qubit used to check DNA and RNA quantity (ng/μl).
TapeStation used to check RNA quality.
DNA Standard 1: 190.13 ng/μl
DNA Standard 2: 21,227.88 ng/μl
RNA Standard 1: 397.54 ng/μl
RNA Standard 2: 10,498.84 ng/μl
| Timepoint | Species | Coral ID | Extraction ID | Homogenization | DNA Reading 1 | DNA Reading 2 | Average DNA ng/μl | RNA Reading 1 | RNA Reading 2 | Average RNA ng/μl | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20180929 | Pocillopora | 1721 | 267 | soft | 34 | 33.8 | 33.9 | 80.4 | 79.4 | 79.9 | 7.6 |
| 20180929 | Pocillopora | 1721 | 268 | hard | 19.4 | 19.3 | 19.35 | 54.8 | 54.8 | 54.8 | 7.6 |
| 20180929 | Pocillopora | 1721 | 269 | soft | 41.4 | 41.4 | 41.4 | 123 | 123 | 123 | 8 |
| 20180929 | Pocillopora | 1721 | 270 | hard | 24.6 | 24.6 | 24.6 | 86.2 | 85.8 | 86 | 8 |
| 20180929 | Montipora | 1114 | 271 | soft | 24.8 | 24.8 | 24.8 | 36 | 36 | 36 | 8.7 |
| 20180929 | Montipora | 1114 | 272 | hard | 10.1 | 10 | 10.05 | 23.6 | 23.6 | 23.6 | 6.4 |
| 20180922 | Pocillopora | 2527 | 273 | soft | 68.2 | 67.8 | 68 | 125 | 125 | 125 | 7.7 |
| 20180922 | Pocillopora | 2527 | 274 | hard | 61.2 | 61.2 | 61.2 | 87.2 | 87.2 | 87.2 | 8.2 |
| 20180922 | Pocillopora | 1637 | 275 | soft | 86.6 | 86.6 | 86.6 | 106 | 106 | 106 | 7.9 |
| 20180922 | Pocillopora | 1637 | 276 | hard | 49.6 | 49.6 | 49.6 | 69.4 | 69.4 | 69.4 | 7.7 |
| 20180922 | Pocillopora | 1637 | 277 | soft | 38 | 37.8 | 37.9 | 82 | 82 | 82 | 8 |
| 20180922 | Pocillopora | 1637 | 278 | hard | 19.7 | 19.6 | 19.65 | 63.8 | 64 | 63.9 | ** |
| 20181020 | Montipora | 1082 | 279 | soft | 12.9 | 12.8 | 12.85 | 14 | 14 | 14 | ** |
| 20181020 | Montipora | 1082 | 280 | hard | 9.18 | 9.1 | 9.14 | 12.2 | 12.2 | 12.2 | 8.2 |
| 20180929 | Montipora | 1212 | 281 | soft | 9.96 | 9.94 | 9.95 | 10.6 | 10.6 | 10.6 | 6.2 |
| 20180929 | Montipora | 1212 | 282 | hard | 7.58 | 7.52 | 7.55 | ** | ** | ** | 7.6 |
Think about re-qubiting 1212 hard 282 and re-tapestation 1637 hard 278.
Link to 20190805 TapeStation report, Extractions #267-282
20190806 E.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here. With the following changes:
- DNA: The first and second elutions were spun down in separate microcentrifuge tubes to compare the quantity and quality between the first and second elution. The first elution had no incubation period (previously 5 minutes) with 10 μl of Tris buffer and the second elution had a 15 minute incubation period (previously 5 minutes) with 100 μl of Tris buffer.
- 10 μl aliquots of DNA and 5 μl aliquots of RNA (instead of 10 μl of each).
Coral fragments were randomly chosen from different timepoint bags.
Fragment preparation start 13:05 end 13:34
Soft/Hard homogenization start 13:35 end 14:25
RNA DNA Extraction start 14:26 end 17:05
Qubit used to check DNA and RNA quantity (ng/μl).
TapeStation used to check RNA quality.
DNA Standard 1: 199.17 ng/μl
DNA Standard 2: 21,160.02 ng/μl
RNA Standard 1: 396.79 ng/μl
RNA Standard 2: 10,606.91 ng/μl
| Timepoint | Species | Coral ID | Extraction ID | Homogenization | DNA Reading 1 | DNA Reading 2 | Average DNA ng/μl | RNA Reading 1 | RNA Reading 2 | Average RNA ng/μl | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|
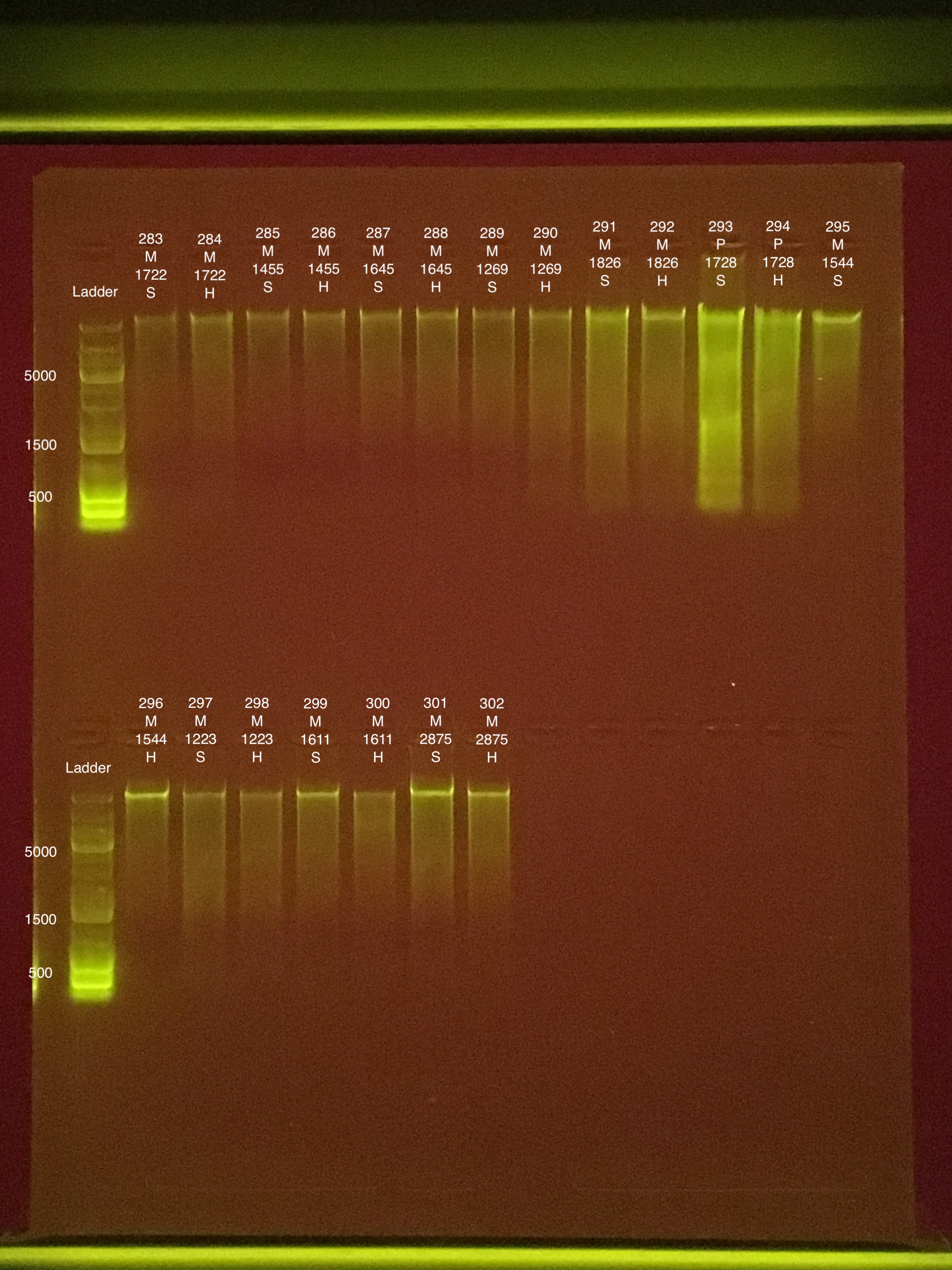
| 20181006 | Montipora | 1722 | 283 | soft | 5.34 | 5.1 | 5.22 | 16.2 | 16 | 16.1 | 8.7 |
| 20181006 | Montipora | 1722 | 284 | hard | 6.78 | 6.8 | 6.79 | 13.8 | 13.6 | 13.7 | 8.4 |
| 20181006 | Montipora | 1455 | 285 | soft | 6.16 | 6.1 | 6.13 | 15.8 | 15.8 | 15.8 | 9.1 |
| 20181006 | Montipora | 1455 | 286 | hard | 5.2 | 5.14 | 5.17 | ** | ** | ** | ** |
| 20181006 | Montipora | 1645 | 287 | soft | 8.94 | 8.88 | 8.91 | 21 | 21 | 21 | 8.6 |
| 20181006 | Montipora | 1645 | 288 | hard | 10.3 | 10.2 | 10.25 | 13.2 | 12.8 | 13 | 9 |
| 20180922 | Montipora | 1269 | 289 | soft | 8.96 | 8.9 | 8.93 | 18 | 18 | 18 | 8.6 |
| 20180922 | Montipora | 1269 | 290 | hard | 11.1 | 11 | 11.05 | 16.2 | 16.2 | 16.2 | 9 |
| 20180922 | Montipora | 1826 | 291 | soft | 18.4 | 18.3 | 18.35 | 25.8 | 25.8 | 25.8 | 8.9 |
| 20180922 | Montipora | 1826 | 292 | hard | 19.1 | 18.9 | 19 | 16.8 | 17 | 16.9 | 9 |
| 20181006 | Pocillopora | 1728 | 293 | soft | 108 | 108 | 108 | 78 | 77.8 | 77.9 | 8.1 |
| 20181006 | Pocillopora | 1728 | 294 | hard | 48.2 | 48 | 48.1 | 47.4 | 47.4 | 47.4 | 8.2 |
| 20180922 | Montipora | 1544 | 295 | soft | 14.9 | 14.8 | 14.85 | 22.4 | 22.2 | 22.3 | 8.8 |
| 20180922 | Montipora | 1544 | 296 | hard | 15.6 | 15.5 | 15.55 | 17 | 16.8 | 16.9 | 8.9 |
| 20181006 | Montipora | 1223 | 297 | soft | 11.9 | 11.9 | 11.9 | 14.4 | 14.4 | 14.4 | 9.2 |
| 20181006 | Montipora | 1223 | 298 | hard | 6.02 | 5.96 | 5.99 | 11.8 | 12 | 11.9 | ** |
| 20180929 | Montipora | 1611 | 299 | soft | 11.5 | 11.4 | 11.45 | 20.4 | 20.4 | 20.4 | 8.8 |
| 20180929 | Montipora | 1611 | 300 | hard | 5.66 | 5.62 | 5.64 | 18.4 | 18.6 | 18.5 | 8.9 |
| 20181006 | Montipora | 2875 | 301 | soft | 29 | 29 | 29 | 26.4 | 26.2 | 26.3 | 8.7 |
| 20181006 | Montipora | 2875 | 302 | hard | 11.4 | 11.3 | 11.35 | 17.6 | 18.2 | 17.9 | 8.7 |
Not enough tapestation tips, only the first 7 samples ran.
Link to 20190806 TapeStation report, Extractions #283-289
20190809 edit:
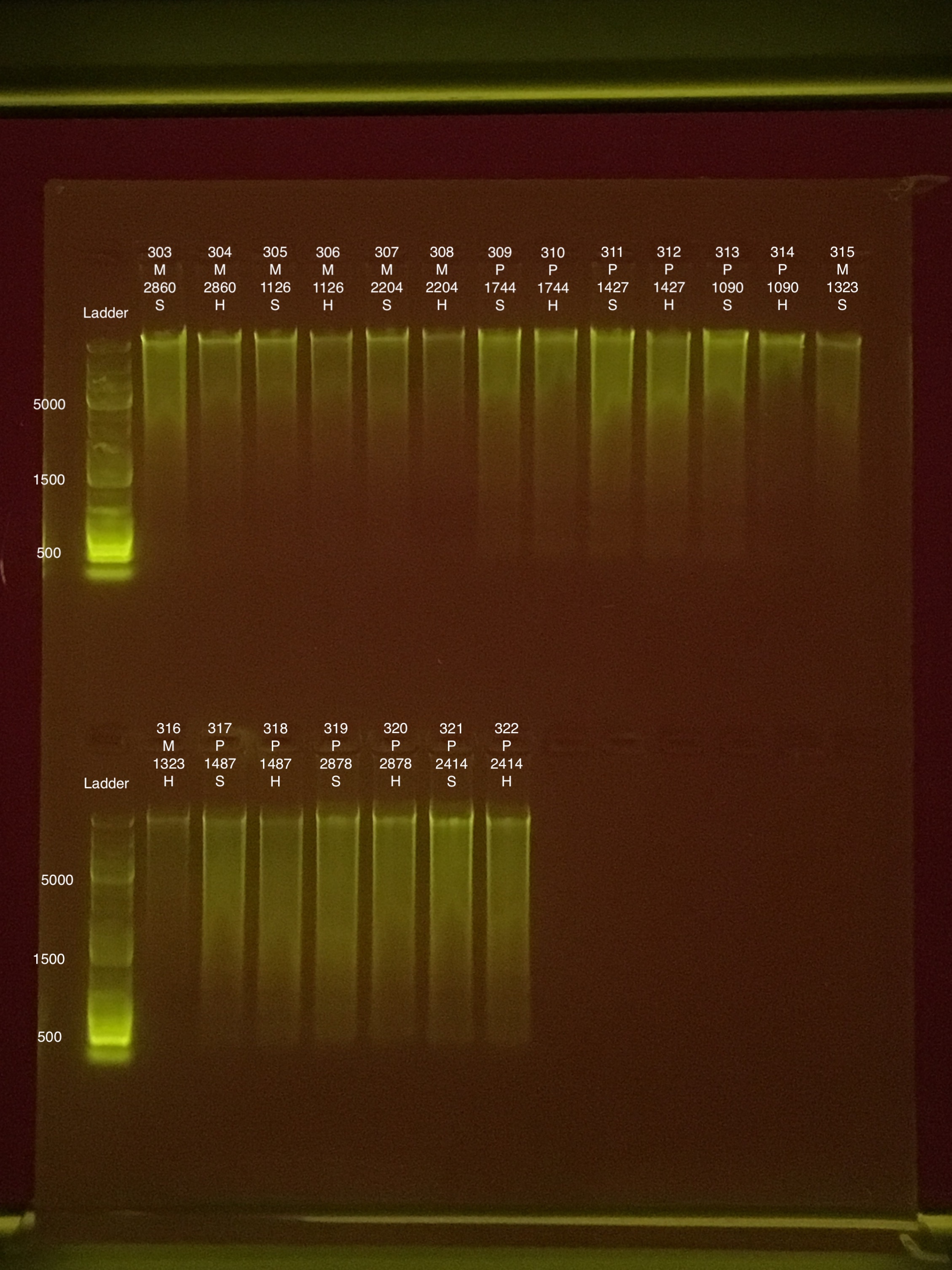
Link to 20190809 TapeStation report, Extractions #290-342
| Coral ID | Extraction ID | RIN |
|---|---|---|
| 1269 | 290 | 9 |
| 1826 | 291 | 8.9 |
| 1826 | 292 | 9 |
| 1728 | 293 | 8.1 |
| 1728 | 294 | 8.2 |
| 1544 | 295 | 8.8 |
| 1544 | 296 | 8.9 |
| 1223 | 297 | 9.2 |
| 1223 | 298 | ** |
| 1611 | 299 | 8.8 |
| 1611 | 300 | 8.9 |
| 2875 | 301 | NA |
| 2875 | 302 | 8.7 |
| 2860 | 303 | 9.1 |
| 2860 | 304 | 9 |
| 1126 | 305 | 8.7 |
| 1126 | 306 | 8.8 |
| 2204 | 307 | 8.6 |
| 2204 | 308 | 9.1 |
| 1744 | 309 | 8.4 |
| 1744 | 310 | 8.2 |
| 1427 | 311 | 8.8 |
| 1427 | 312 | 8.5 |
| 1090 | 313 | NA |
| 1090 | 314 | 8.1 |
| 1323 | 315 | 9.2 |
| 1323 | 316 | ** |
| 1487 | 317 | 7.4 |
| 1487 | 318 | 7.3 |
| 2878 | 319 | 8.2 |
| 2878 | 320 | 7.9 |
| 2414 | 321 | 8.6 |
| 2414 | 322 | 8.2 |
| 2409 | 323 | 8.2 |
| 2409 | 324 | 7.8 |
| 1047 | 325 | NA |
| 1047 | 326 | 7.8 |
| 1595 | 327 | 8.5 |
| 1595 | 328 | 8.6 |
| 1499 | 329 | 9 |
| 1499 | 330 | 8.7 |
| 2068 | 331 | 8.7 |
| 2068 | 332 | ** |
Extractions #: 301, 313, 325, and 337 did not run because not enough TapeStation screentapes.
20190812 edit:
TapeStation ran for Extraction ID #s: 301, 313, 325, and 337.
Link to 20190812 TapeStation report, Extractions #301, 313, 325, 337
20190807 E.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here. With the following changes:
- DNA: The first and second elutions were spun down in separate microcentrifuge tubes to compare the quantity and quality between the first and second elution. The first elution had no incubation period (previously 5 minutes) with 10 μl of Tris buffer and the second elution had a 15 minute incubation period (previously 5 minutes) with 100 μl of Tris buffer.
- 10 μl aliquots of DNA and 5 μl aliquots of RNA (instead of 10 μl of each).
Coral fragments were randomly chosen from different timepoint bags.
Fragment preparation start 11:28 end 12:07
Soft/Hard homogenization start 12:08 end NA
RNA DNA Extraction start NA end 15:15
Qubit used to check DNA and RNA quantity (ng/μl).
TapeStation used to check RNA quality.
DNA Standard 1: 193.68 ng/μl
DNA Standard 2: 19,619.20 ng/μl
RNA Standard 1: 390.76 ng/μl
RNA Standard 2: 10,145.65 ng/μl
| Timepoint | Species | Coral ID | Extraction ID | Homogenization | DNA Reading 1 | DNA Reading 2 | Average DNA ng/μl | RNA Reading 1 | RNA Reading 2 | Average RNA ng/μl | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20181006 | Montipora | 2860 | 303 | soft | 43.8 | 43.2 | 43.5 | 45.2 | 45 | 45.1 | 9.1 |
| 20181006 | Montipora | 2860 | 304 | hard | 17.8 | 17.7 | 17.75 | 23.8 | 23.6 | 23.7 | 9 |
| 20181006 | Montipora | 1126 | 305 | soft | 19.1 | 19.1 | 19.1 | 30.8 | 30.6 | 30.7 | 8.7 |
| 20181006 | Montipora | 1126 | 306 | hard | 13.4 | 13.4 | 13.4 | 16.6 | 16.6 | 16.6 | 8.8 |
| 20180922 | Montipora | 2204 | 307 | soft | 21.4 | 21.4 | 21.4 | 41.4 | 41.4 | 41.4 | 8.6 |
| 20180922 | Montipora | 2204 | 308 | hard | 11.5 | 11.4 | 11.45 | 33.6 | 33.2 | 33.4 | 9.1 |
| 20180929 | Pocillopora | 1744 | 309 | soft | 27.4 | 27.4 | 27.4 | 83.8 | 83.4 | 83.6 | 8.4 |
| 20180929 | Pocillopora | 1744 | 310 | hard | 21.8 | 21.8 | 21.8 | 40.4 | 40.2 | 40.3 | 8.2 |
| 20181006 | Pocillopora | 1427 | 311 | soft | 43 | 42.8 | 42.9 | 52.4 | 52.2 | 52.3 | 8.8 |
| 20181006 | Pocillopora | 1427 | 312 | hard | 29.8 | 29.6 | 29.7 | 34.4 | 34.2 | 34.3 | 8.5 |
| 20181006 | Pocillopora | 1090 | 313 | soft | 31.6 | 31.4 | 31.5 | 30.6 | 30.6 | 30.6 | 8.4 |
| 20181006 | Pocillopora | 1090 | 314 | hard | 14 | 14 | 14 | 25.4 | 25.4 | 25.4 | 8.1 |
| 20180922 | Montipora | 1323 | 315 | soft | 19.5 | 19.5 | 19.5 | 23.4 | 23.4 | 23.4 | 9.2 |
| 20180922 | Montipora | 1323 | 316 | hard | 10.8 | 10.7 | 10.75 | 17 | 17 | 17 | ** |
| 20181006 | Pocillopora | 1487 | 317 | soft | 37.4 | 37.2 | 37.3 | 69 | 68.6 | 68.8 | 7.4 |
| 20181006 | Pocillopora | 1487 | 318 | hard | 36.2 | 36 | 36.1 | 34 | 33.8 | 33.9 | 7.3 |
| 20181006 | Pocillopora | 2878 | 319 | soft | 48.2 | 48 | 48.1 | 59 | 58.8 | 58.9 | 8.2 |
| 20181006 | Pocillopora | 2878 | 320 | hard | 48.8 | 48.6 | 48.7 | 50.4 | 50.2 | 50.3 | 7.9 |
| 20180922 | Pocillopora | 2414 | 321 | soft | 63.8 | 63.6 | 63.7 | 112 | 112 | 112 | 8.6 |
| 20180922 | Pocillopora | 2414 | 322 | hard | 48 | 48 | 48 | 59.4 | 59.2 | 59.3 | 8.2 |
Not enough TapeStation tips to run today’s samples.
20190809 edit:
Link to 20190809 TapeStation report, Extractions #290-342
| Coral ID | Extraction ID | RIN |
|---|---|---|
| 1269 | 290 | 9 |
| 1826 | 291 | 8.9 |
| 1826 | 292 | 9 |
| 1728 | 293 | 8.1 |
| 1728 | 294 | 8.2 |
| 1544 | 295 | 8.8 |
| 1544 | 296 | 8.9 |
| 1223 | 297 | 9.2 |
| 1223 | 298 | ** |
| 1611 | 299 | 8.8 |
| 1611 | 300 | 8.9 |
| 2875 | 301 | NA |
| 2875 | 302 | 8.7 |
| 2860 | 303 | 9.1 |
| 2860 | 304 | 9 |
| 1126 | 305 | 8.7 |
| 1126 | 306 | 8.8 |
| 2204 | 307 | 8.6 |
| 2204 | 308 | 9.1 |
| 1744 | 309 | 8.4 |
| 1744 | 310 | 8.2 |
| 1427 | 311 | 8.8 |
| 1427 | 312 | 8.5 |
| 1090 | 313 | NA |
| 1090 | 314 | 8.1 |
| 1323 | 315 | 9.2 |
| 1323 | 316 | ** |
| 1487 | 317 | 7.4 |
| 1487 | 318 | 7.3 |
| 2878 | 319 | 8.2 |
| 2878 | 320 | 7.9 |
| 2414 | 321 | 8.6 |
| 2414 | 322 | 8.2 |
| 2409 | 323 | 8.2 |
| 2409 | 324 | 7.8 |
| 1047 | 325 | NA |
| 1047 | 326 | 7.8 |
| 1595 | 327 | 8.5 |
| 1595 | 328 | 8.6 |
| 1499 | 329 | 9 |
| 1499 | 330 | 8.7 |
| 2068 | 331 | 8.7 |
| 2068 | 332 | ** |
Extractions #: 301, 313, 325, and 337 did not run because not enough TapeStation screentapes.
20190812 edit:
TapeStation ran for Extraction ID #s: 301, 313, 325, and 337.
Link to 20190812 TapeStation report, Extractions #301, 313, 325, 337
20190808 E.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here. With the following changes:
- DNA: The first and second elutions were spun down in separate microcentrifuge tubes to compare the quantity and quality between the first and second elution. The first elution had no incubation period (previously 5 minutes) with 10 μl of Tris buffer and the second elution had a 15 minute incubation period (previously 5 minutes) with 100 μl of Tris buffer.
- 10 μl aliquots of DNA and 5 μl aliquots of RNA (instead of 10 μl of each).
Coral fragments were randomly chosen from different timepoint bags.
Qubit used to check DNA and RNA quantity (ng/μl).
TapeStation used to check RNA quality.
DNA Standard 1: 194.56 ng/μl
DNA Standard 2: 20,715.31 ng/μl
RNA Standard 1: 400.38 ng/μl
RNA Standard 2: 10,582.65 ng/μl
| Timepoint | Species | Coral ID | Extraction ID | Homogenization | DNA Reading 1 | DNA Reading 2 | Average DNA ng/μl | RNA Reading 1 | RNA Reading 2 | Average RNA ng/μl | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|
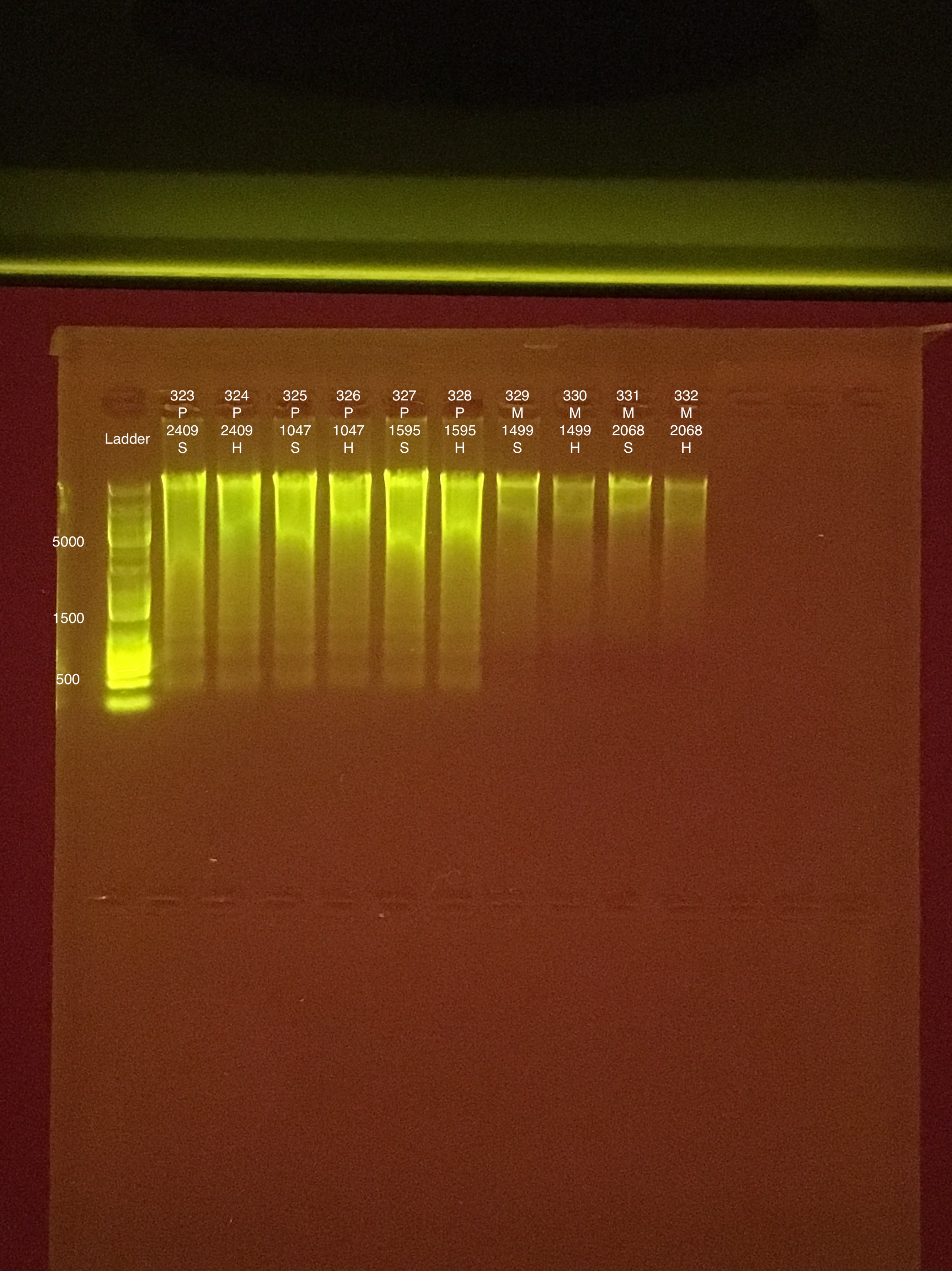
| 20181006 | Pocillopora | 2409 | 323 | soft | 73.8 | 73.2 | 73.5 | 46.2 | 46 | 46.1 | 8.2 |
| 20181006 | Pocillopora | 2409 | 324 | hard | 55.8 | 55.6 | 55.7 | 50 | 49.8 | 49.9 | 7.8 |
| 20181006 | Pocillopora | 1047 | 325 | soft | 57.6 | 57.4 | 57.5 | 88.6 | 88.2 | 88.4 | 8.3 |
| 20181006 | Pocillopora | 1047 | 326 | hard | 37.4 | 37.4 | 37.4 | 30.8 | 30.8 | 30.8 | 7.8 |
| 20180929 | Pocillopora | 1595 | 327 | soft | 88.2 | 88 | 88.1 | 96.4 | 95.8 | 96.1 | 8.5 |
| 20180929 | Pocillopora | 1595 | 328 | hard | 57.8 | 57.4 | 57.6 | 66.2 | 66 | 66.1 | 8.6 |
| 20181006 | Montipora | 1499 | 329 | soft | 27.6 | 27.4 | 27.5 | 19.4 | 19.4 | 19.4 | 9 |
| 20181006 | Montipora | 1499 | 330 | hard | 14.4 | 14.3 | 14.35 | 18 | 18 | 18 | 8.7 |
| 20180922 | Montipora | 2068 | 331 | soft | 18 | 17.9 | 17.95 | 15.4 | 15.4 | 15.4 | 8.7 |
| 20180922 | Montipora | 2068 | 332 | hard | 11.2 | 11.1 | 11.15 | 15.6 | 15.6 | 15.6 | ** |
Not enough TapeStation tips to run today’s samples.
20190809 edit:
Link to 20190809 TapeStation report, Extractions #290-342
| Coral ID | Extraction ID | RIN |
|---|---|---|
| 1269 | 290 | 9 |
| 1826 | 291 | 8.9 |
| 1826 | 292 | 9 |
| 1728 | 293 | 8.1 |
| 1728 | 294 | 8.2 |
| 1544 | 295 | 8.8 |
| 1544 | 296 | 8.9 |
| 1223 | 297 | 9.2 |
| 1223 | 298 | ** |
| 1611 | 299 | 8.8 |
| 1611 | 300 | 8.9 |
| 2875 | 301 | NA |
| 2875 | 302 | 8.7 |
| 2860 | 303 | 9.1 |
| 2860 | 304 | 9 |
| 1126 | 305 | 8.7 |
| 1126 | 306 | 8.8 |
| 2204 | 307 | 8.6 |
| 2204 | 308 | 9.1 |
| 1744 | 309 | 8.4 |
| 1744 | 310 | 8.2 |
| 1427 | 311 | 8.8 |
| 1427 | 312 | 8.5 |
| 1090 | 313 | NA |
| 1090 | 314 | 8.1 |
| 1323 | 315 | 9.2 |
| 1323 | 316 | ** |
| 1487 | 317 | 7.4 |
| 1487 | 318 | 7.3 |
| 2878 | 319 | 8.2 |
| 2878 | 320 | 7.9 |
| 2414 | 321 | 8.6 |
| 2414 | 322 | 8.2 |
| 2409 | 323 | 8.2 |
| 2409 | 324 | 7.8 |
| 1047 | 325 | NA |
| 1047 | 326 | 7.8 |
| 1595 | 327 | 8.5 |
| 1595 | 328 | 8.6 |
| 1499 | 329 | 9 |
| 1499 | 330 | 8.7 |
| 2068 | 331 | 8.7 |
| 2068 | 332 | ** |
Extractions #: 301, 313, 325, and 337 did not run because not enough TapeStation screentapes.
20190813 edit:
Link to 20190812 TapeStation report, Extractions #301, 313, 325, 337
Extraction ID #325: 8.3
20190809 E.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here. With the following changes:
- DNA: The first and second elutions were spun down in separate microcentrifuge tubes to compare the quantity and quality between the first and second elution. The first elution had no incubation period (previously 5 minutes) with 10 μl of Tris buffer and the second elution had a 15 minute incubation period (previously 5 minutes) with 100 μl of Tris buffer.
- 10 μl aliquots of DNA and 5 μl aliquots of RNA (instead of 10 μl of each).
Coral fragments were randomly chosen from different timepoint bags.
Qubit used to check DNA and RNA quantity (ng/μl).
TapeStation used to check RNA quality.
DNA Standard 1: 188.61 ng/μl
DNA Standard 2: 19,960.99 ng/μl
RNA Standard 1: 396.14 ng/μl
RNA Standard 2: 10,702 ng/μl
| Timepoint | Species | Coral ID | Extraction ID | Homogenization | DNA Reading 1 | DNA Reading 2 | Average DNA ng/μl | RNA Reading 1 | RNA Reading 2 | Average RNA ng/μl | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|
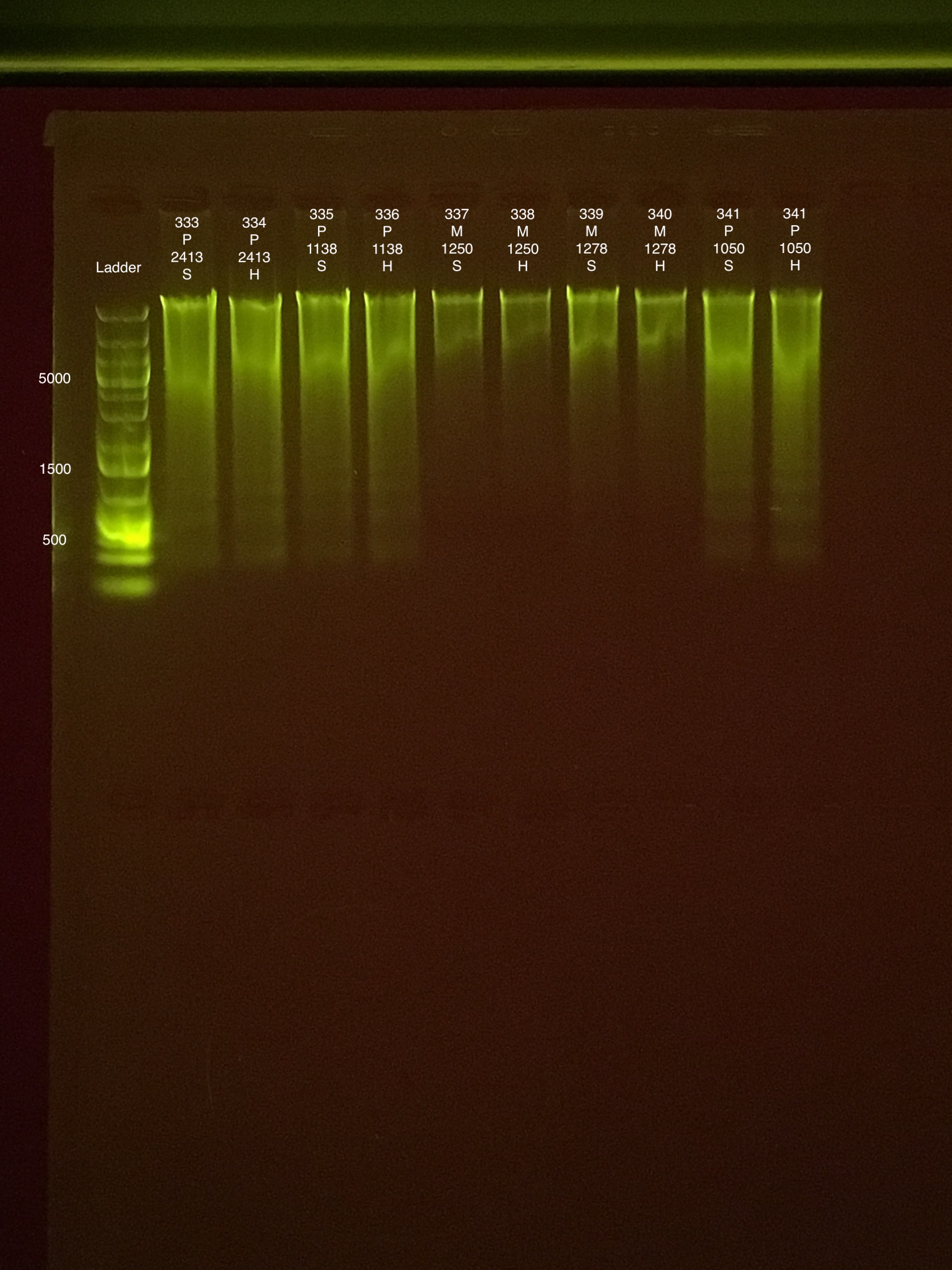
| 20181006 | Pocillopora | 2413 | 333 | soft | 59.2 | 59 | 59.1 | 71 | 70.8 | 70.9 | 8.5 |
| 20181006 | Pocillopora | 2413 | 334 | hard | 37 | 36.8 | 36.9 | 44 | 43.8 | 43.9 | 8 |
| 20180929 | Pocillopora | 1138 | 335 | soft | 47.4 | 47 | 47.2 | 59.8 | 58.6 | 59.2 | 8.8 |
| 20180929 | Pocillopora | 1138 | 336 | hard | 33.2 | 33 | 33.1 | 33.6 | 33.4 | 33.5 | 8.2 |
| 20181006 | Montipora | 1250 | 337 | soft | 16.2 | 16.2 | 16.2 | 15 | 15 | 15 | 9.1 |
| 20181006 | Montipora | 1250 | 338 | hard | 12.5 | 12.5 | 12.5 | 12.4 | 12.4 | 12.4 | ** |
| 20181006 | Montipora | 1278 | 339 | soft | 32.6 | 32.6 | 32.6 | 25 | 25 | 25 | 8.8 |
| 20181006 | Montipora | 1278 | 340 | hard | 19.1 | 19.1 | 19.1 | 17.8 | 17.8 | 17.8 | 8.7 |
| 20180929 | Pocillopora | 1050 | 341 | soft | 38.6 | 38.2 | 38.4 | 86 | 85.8 | 85.9 | 7.6 |
| 20180929 | Pocillopora | 1050 | 342 | hard | 35.4 | 35.4 | 35.4 | 59.2 | 59.2 | 59.2 | 7.2 |
Link to 20190809 TapeStation report, Extractions #290-342
Link to 20190812 TapeStation report, Extractions #301, 313, 325, 337
| Coral ID | Extraction ID | RIN |
|---|---|---|
| 1269 | 290 | 9 |
| 1826 | 291 | 8.9 |
| 1826 | 292 | 9 |
| 1728 | 293 | 8.1 |
| 1728 | 294 | 8.2 |
| 1544 | 295 | 8.8 |
| 1544 | 296 | 8.9 |
| 1223 | 297 | 9.2 |
| 1223 | 298 | ** |
| 1611 | 299 | 8.8 |
| 1611 | 300 | 8.9 |
| 2875 | 301 | NA |
| 2875 | 302 | 8.7 |
| 2860 | 303 | 9.1 |
| 2860 | 304 | 9 |
| 1126 | 305 | 8.7 |
| 1126 | 306 | 8.8 |
| 2204 | 307 | 8.6 |
| 2204 | 308 | 9.1 |
| 1744 | 309 | 8.4 |
| 1744 | 310 | 8.2 |
| 1427 | 311 | 8.8 |
| 1427 | 312 | 8.5 |
| 1090 | 313 | NA |
| 1090 | 314 | 8.1 |
| 1323 | 315 | 9.2 |
| 1323 | 316 | ** |
| 1487 | 317 | 7.4 |
| 1487 | 318 | 7.3 |
| 2878 | 319 | 8.2 |
| 2878 | 320 | 7.9 |
| 2414 | 321 | 8.6 |
| 2414 | 322 | 8.2 |
| 2409 | 323 | 8.2 |
| 2409 | 324 | 7.8 |
| 1047 | 325 | NA |
| 1047 | 326 | 7.8 |
| 1595 | 327 | 8.5 |
| 1595 | 328 | 8.6 |
| 1499 | 329 | 9 |
| 1499 | 330 | 8.7 |
| 2068 | 331 | 8.7 |
| 2068 | 332 | ** |
Extractions #: 301, 313, 325, and 337 did not run because not enough TapeStation screentapes.
20190813 edit:
TapeStation ran for Extraction IDs #s: 301, 313, 325, 337. See above for tapestation report link. Extraction ID # 337: 9.1
20190814 M.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here. With the following changes:
- DNA: The first and second elutions were spun down in separate microcentrifuge tubes to compare the quantity and quality between the first and second elution. The first elution had no incubation period (previously 5 minutes) with 10 μl of Tris buffer and the second elution had a 15 minute incubation period (previously 5 minutes) with 100 μl of Tris buffer.
- 10 μl aliquots of DNA and 5 μl aliquots of RNA (instead of 10 μl of each).
Coral fragments were randomly chosen from different timepoint bags.
Qubit used to check DNA and RNA quantity (ng/μl).
TapeStation used to check RNA quality.
DNA Standard 1: 185.60 ng/μl
DNA Standard 2: 20,540.05 ng/μl
RNA Standard 1: 386.59 ng/μl
RNA Standard 2: 10,597.72 ng/μl
| Timepoint | Species | Coral ID | Extraction ID | Homogenization | DNA Reading 1 | DNA Reading 2 | Average DNA ng/μl | RNA Reading 1 | RNA Reading 2 | Average RNA ng/μl | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|
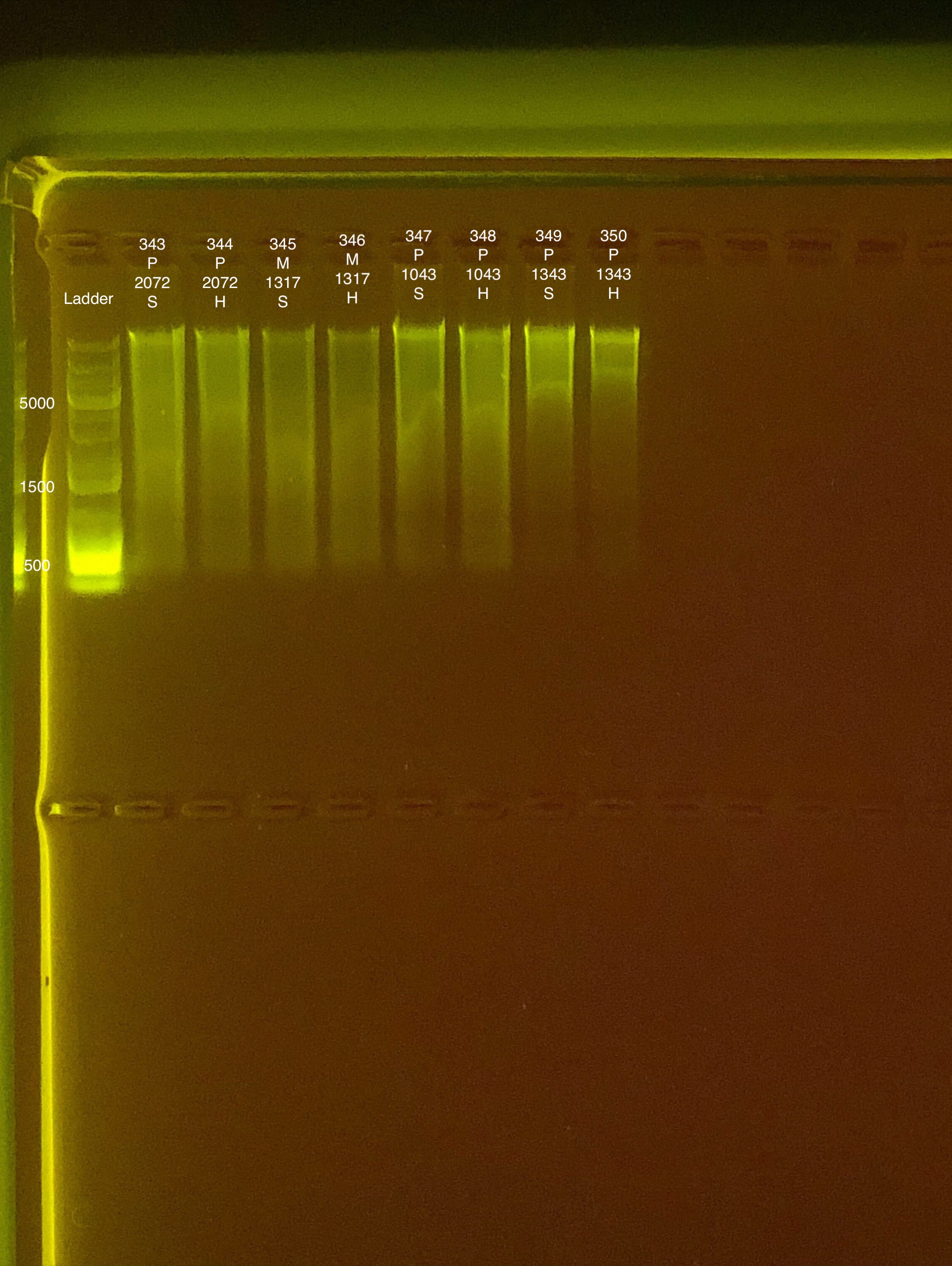
| 20181006 | Pocillopora | 2072 | 343 | soft | 41.4 | 41.2 | 41.3 | 59.6 | 59.6 | 59.6 | 6.6 |
| 20181006 | Pocillopora | 2072 | 344 | hard | 20.2 | 20.2 | 20.2 | 24.6 | 24.6 | 24.6 | NA |
| 20180929 | Montipora | 1317 | 345 | soft | 19.2 | 19.1 | 19.15 | 23.4 | 23.4 | 23.4 | 7.7 |
| 20180929 | Montipora | 1317 | 346 | hard | 12.2 | 12.1 | 12.15 | 14.4 | 14.4 | 14.4 | NA |
| 20180922 | Pocillopora | 1043 | 347 | soft | 43.8 | 43.8 | 43.8 | 55 | 55.2 | 55.1 | 8.1 |
| 20180922 | Pocillopora | 1043 | 348 | hard | 26 | 25.8 | 25.9 | 37.6 | 37.8 | 37.7 | NA |
| 20180922 | Pocillopora | 1343 | 349 | soft | 23.8 | 23.8 | 23.8 | 33.2 | 33.4 | 33.3 | 8.4 |
| 20180922 | Pocillopora | 1343 | 350 | hard | 11.7 | 11.7 | 11.7 | 24.6 | 24.6 | 24.6 | NA |
Link to 20190814 TapeStation report, Extractions #343-350
20190815 M.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here. With the following changes:
- DNA: The first and second elutions were spun down in separate microcentrifuge tubes to compare the quantity and quality between the first and second elution. The first elution had no incubation period (previously 5 minutes) with 10 μl of Tris buffer and the second elution had a 15 minute incubation period (previously 5 minutes) with 100 μl of Tris buffer.
- 10 μl aliquots of DNA and 5 μl aliquots of RNA (instead of 10 μl of each).
Coral fragments were randomly chosen from different timepoint bags.
Qubit used to check DNA and RNA quantity (ng/μl).
TapeStation used to check RNA quality.
DNA Standard 1: 170.27 ng/μl
DNA Standard 2: 18,071.08 ng/μl
RNA Standard 1: 390.40 ng/μl
RNA Standard 2: 10,072.23 ng/μl
| Timepoint | Species | Coral ID | Extraction ID | Homogenization | DNA Reading 1 | DNA Reading 2 | Average DNA ng/μl | RNA Reading 1 | RNA Reading 2 | Average RNA ng/μl | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|
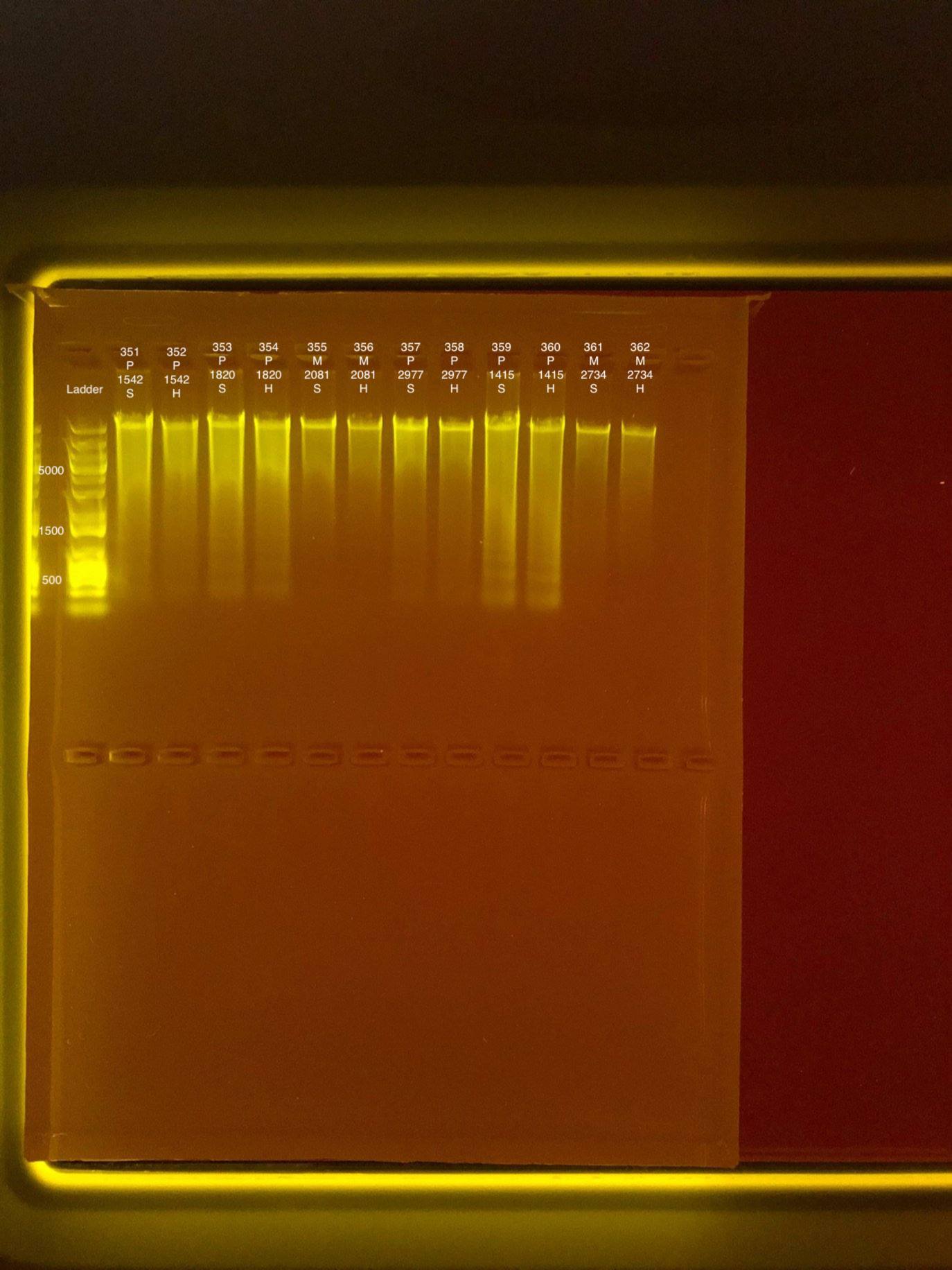
| 20180929 | Pocillopora | 1542 | 351 | soft | 38 | 37.8 | 37.9 | 38.2 | 38.2 | 38.2 | 8.1 |
| 20180929 | Pocillopora | 1542 | 352 | hard | 15.4 | 15.3 | 15.35 | 29 | 29 | 29 | NA |
| 20181006 | Pocillopora | 1820 | 353 | soft | 35.4 | 35.4 | 35.4 | 46.4 | 46.2 | 46.3 | 8.3 |
| 20181006 | Pocillopora | 1820 | 354 | hard | 21.4 | 21.4 | 21.4 | 23.6 | 23.6 | 23.6 | NA |
| 20180922 | Montipora | 2081 | 355 | soft | 15.7 | 15.7 | 15.7 | 17.8 | 17.8 | 17.8 | 8.1 |
| 20180922 | Montipora | 2081 | 356 | hard | 10.4 | 10.4 | 10.4 | 14.4 | 14.4 | 14.4 | NA |
| 20180922 | Pocillopora | 2977 | 357 | soft | 21.2 | 21.2 | 21.2 | 49 | 49.2 | 49.1 | 8.6 |
| 20180922 | Pocillopora | 2977 | 358 | hard | 14.8 | 14.7 | 14.75 | 38.2 | 38.2 | 38.2 | NA |
| 20180923 | Pocillopora | 1415 | 359 | soft | 53.6 | 53.6 | 53.6 | 54.8 | 55 | 54.9 | 8.4 |
| 20180923 | Pocillopora | 1415 | 360 | hard | 36.4 | 36.4 | 36.4 | 38.4 | 38.4 | 38.4 | NA |
| 20180922 | Montipora | 2734 | 361 | soft | 7.88 | 7.82 | 7.85 | ** | ** | ** | ** |
| 20180922 | Montipora | 2734 | 362 | hard | 7.4 | 7.34 | 7.37 | ** | ** | ** | NA |
Link to 20190815 TapeStation report, Extractions #351-362
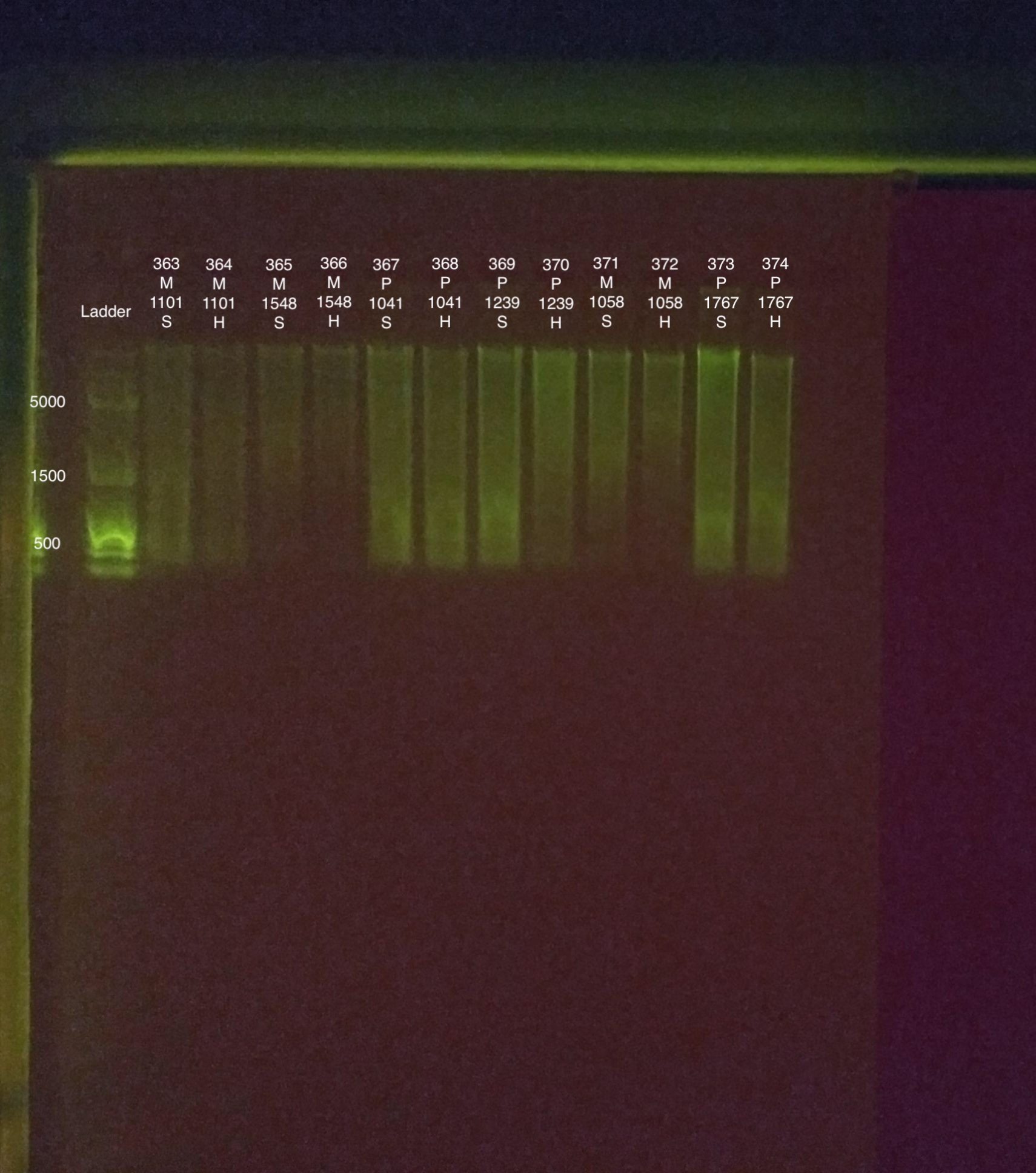
20190823 M.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here. With the following changes:
- DNA: The first and second elutions were spun down in separate microcentrifuge tubes to compare the quantity and quality between the first and second elution. The first elution had no incubation period (previously 5 minutes) with 10 μl of Tris buffer and the second elution had a 15 minute incubation period (previously 5 minutes) with 100 μl of Tris buffer.
- 10 μl aliquots of DNA and 5 μl aliquots of RNA (instead of 10 μl of each).
Coral fragments were randomly chosen from different timepoint bags.
Qubit used to check DNA and RNA quantity (ng/μl).
TapeStation used to check RNA quality.
DNA Standard 1: 157.18 ng/μl
DNA Standard 2: 16,184.60 ng/μl
RNA Standard 1: 374.21 ng/μl
RNA Standard 2: 10,011.72 ng/μl
| Timepoint | Species | Coral ID | Extraction ID | Homogenization | DNA Reading 1 | DNA Reading 2 | Average DNA ng/μl | RNA Reading 1 | RNA Reading 2 | Average RNA ng/μl | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20180922 | Montipora | 1101 | 363 | soft | 27.2 | 27 | 27.1 | 30.8 | 30.8 | 30.8 | 8.3 |
| 20180922 | Montipora | 1101 | 364 | hard | 13.8 | 13.8 | 13.8 | 19 | 19 | 19 | NA |
| 20180922 | Montipora | 1548 | 365 | soft | 15.6 | 15.6 | 15.6 | 27 | 27 | 27 | 8.4 |
| 20180922 | Montipora | 1548 | 366 | hard | 11.5 | 11.4 | 11.45 | 18.2 | 18.2 | 18.2 | NA |
| 20180922 | Pocillopora | 1041 | 367 | soft | 62.4 | 62.4 | 62.4 | 83.8 | 83.8 | 83.8 | 7.7 |
| 20180922 | Pocillopora | 1041 | 368 | hard | 44 | 44 | 44 | 64.8 | 65 | 64.9 | NA |
| 20180922 | Pocillopora | 1239 | 369 | soft | 77 | 76.8 | 76.9 | 93.4 | 93.4 | 93.4 | 6.9 |
| 20180922 | Pocillopora | 1239 | 370 | hard | 28.6 | 28.6 | 28.6 | 46.2 | 46.2 | 46.2 | NA |
| 20181006 | Montipora | 1058 | 371 | soft | 30.8 | 30.6 | 30.7 | 29.8 | 29.8 | 29.8 | 8.8 |
| 20181006 | Montipora | 1058 | 372 | hard | 15 | 14.9 | 14.95 | 20 | 20 | 20 | NA |
| 20180922 | Pocillopora | 1767 | 373 | soft | 110 | 110 | 110 | 130 | 130 | 130 | 7.3 |
| 20180922 | Pocillopora | 1767 | 374 | hard | 52.2 | 52 | 52.1 | 74.6 | 74.6 | 74.6 | NA |
Link to 20190823 TapeStation report, Extractions #363-374
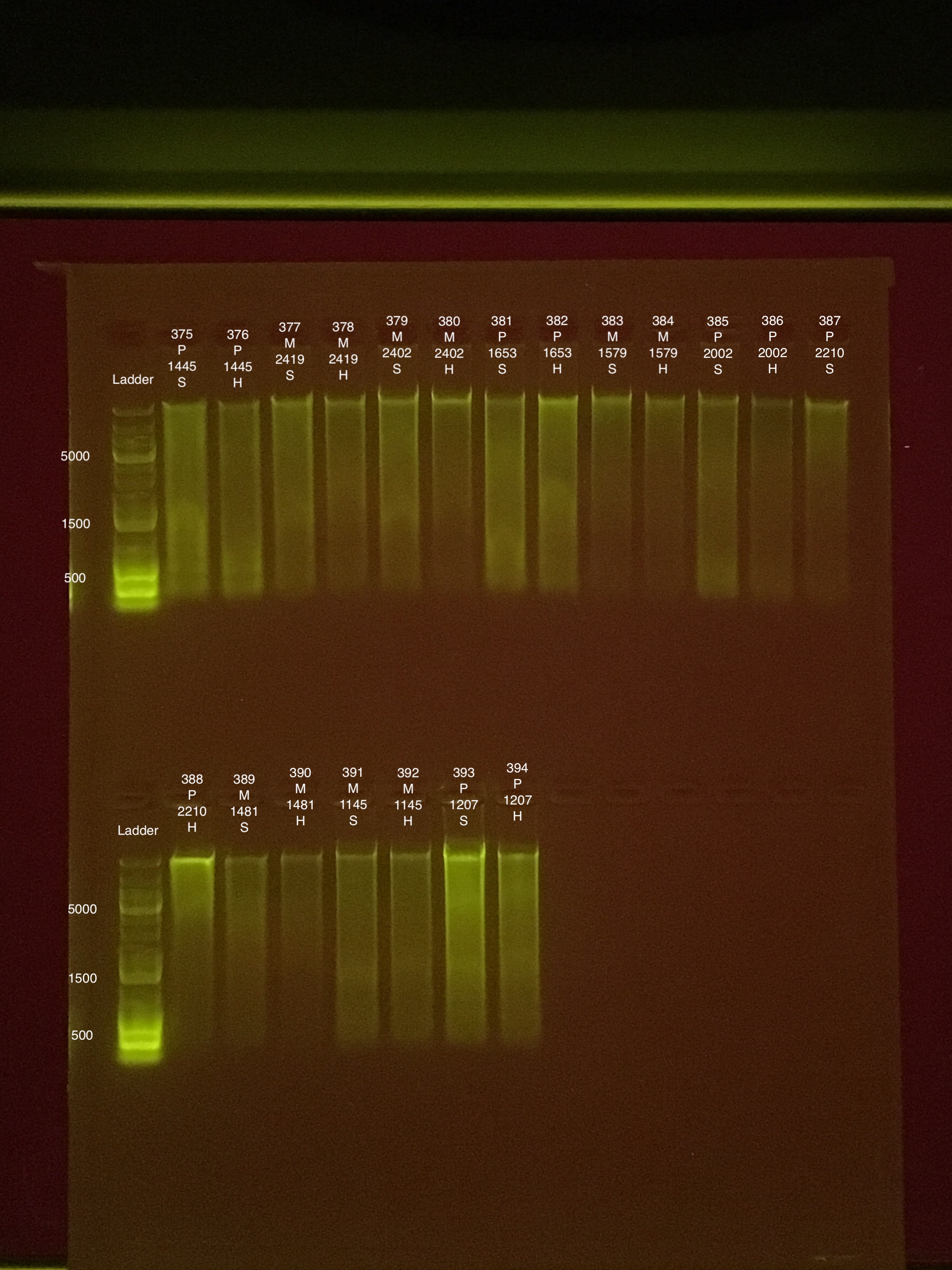
20190826 E.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here. With the following changes:
- DNA: The first and second elutions were spun down in separate microcentrifuge tubes to compare the quantity and quality between the first and second elution. The first elution had no incubation period (previously 5 minutes) with 10 μl of Tris buffer and the second elution had a 15 minute incubation period (previously 5 minutes) with 100 μl of Tris buffer.
- 10 μl aliquots of DNA and 5 μl aliquots of RNA (instead of 10 μl of each).
Coral fragments were randomly chosen from different timepoint bags.
Qubit used to check DNA and RNA quantity (ng/μl).
TapeStation used to check RNA quality.
DNA Standard 1: 202.97 ng/μl
DNA Standard 2: 22,380.18 ng/μl
RNA Standard 1: 394.22 ng/μl
RNA Standard 2: 10,181.09 ng/μl
| Timepoint | Species | Coral ID | Extraction ID | Homogenization | DNA Reading 1 | DNA Reading 2 | Average DNA ng/μl | RNA Reading 1 | RNA Reading 2 | Average RNA ng/μl | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20181006 | Pocillopora | 1445 | 375 | soft | 41.6 | 41.2 | 41.4 | 83.4 | 83.2 | 83.3 | 7.5 |
| 20181006 | Pocillopora | 1445 | 376 | hard | 26.4 | 25.8 | 26.1 | 58.4 | 58.2 | 58.3 | NA |
| 20181006 | Montipora | 2419 | 377 | soft | 26 | 25.8 | 25.9 | 39 | 38.8 | 38.9 | 8.2 |
| 20181006 | Montipora | 2419 | 378 | hard | 15.2 | 15.1 | 15.15 | 17.2 | 17.2 | 17.2 | NA |
| 20180929 | Montipora | 2402 | 379 | soft | 28 | 27.8 | 27.9 | 32.4 | 32.2 | 32.3 | 8.9 |
| 20180929 | Montipora | 2402 | 380 | hard | 18.7 | 18.7 | 18.7 | 14 | 14 | 14 | NA |
| 20180922 | Pocillopora | 1653 | 381 | soft | 44.8 | 44.8 | 44.8 | 114 | 114 | 114 | 6.2 |
| 20180922 | Pocillopora | 1653 | 382 | hard | 32 | 31.8 | 31.9 | 68 | 68 | 68 | NA |
| 20180922 | Montipora | 1579 | 383 | soft | 28.6 | 28.4 | 28.5 | 26.6 | 26.8 | 26.7 | 8.5 |
| 20180922 | Montipora | 1579 | 384 | hard | 16.5 | 16.4 | 16.45 | 15.8 | 16.4 | 16.1 | NA |
| 20180922 | Pocillopora | 2002 | 385 | soft | 48.2 | 48.2 | 48.2 | 80.6 | 80.4 | 80.5 | 7.1 |
| 20180922 | Pocillopora | 2002 | 386 | hard | 26.6 | 26.4 | 26.5 | 53.8 | 53.6 | 53.7 | NA |
| 20180922 | Pocillopora | 2210 | 387 | soft | 29.6 | 29.4 | 29.5 | 70.4 | 70.4 | 70.4 | 7.8 |
| 20180922 | Pocillopora | 2210 | 388 | hard | 21.4 | 21.4 | 21.4 | 50.6 | 50.6 | 50.6 | NA |
| 20180922 | Montipora | 1481 | 389 | soft | 9.5 | 9.46 | 9.48 | 11.8 | 11.8 | 11.8 | ** |
| 20180922 | Montipora | 1481 | 390 | hard | 7.38 | 7.34 | 7.36 | ** | ** | ** | NA |
| 20180922 | Montipora | 1145 | 391 | soft | 25 | 24.8 | 24.9 | 31.6 | 31.6 | 31.6 | 8.3 |
| 20180922 | Montipora | 1145 | 392 | hard | 14.7 | 14.7 | 14.7 | 25.6 | 25.6 | 25.6 | NA |
| 20180922 | Pocillopora | 1207 | 393 | soft | 85 | 84.6 | 84.8 | 114 | 113 | 113.5 | 8.1 |
| 20180922 | Pocillopora | 1207 | 394 | hard | 36 | 35.8 | 35.9 | 44.6 | 44.6 | 44.6 | NA |
Link to 20190827 TapeStation report, Extractions #375-394
20190827 E.S.
DNA/RNA Extractions from Montipora capitata and Pocillopora acuta adult coral fragments from Holobiont Integration Hawaii 2018 project.
Soft and Hard homogenization, and DNA/RNA Extractions followed this protocol. General Zymo Duet DNA/RNA Extraction protocol found here. With the following changes:
- DNA: The first and second elutions were spun down in separate microcentrifuge tubes to compare the quantity and quality between the first and second elution. The first elution had no incubation period (previously 5 minutes) with 10 μl of Tris buffer and the second elution had a 15 minute incubation period (previously 5 minutes) with 100 μl of Tris buffer.
- 10 μl aliquots of DNA and 5 μl aliquots of RNA (instead of 10 μl of each).
Coral fragments were randomly chosen from different timepoint bags.
Extraction numbers #399 and #400 not used. No corals or tubes assigned to #399 or #400.
Qubit used to check DNA and RNA quantity (ng/μl).
TapeStation used to check RNA quality.
DNA Standard 1: 183.02 ng/μl
DNA Standard 2: 22,986.92 ng/μl
RNA Standard 1: 410.43 ng/μl
RNA Standard 2: 10,577.53 ng/μl
| Timepoint | Species | Coral ID | Extraction ID | Homogenization | DNA Reading 1 | DNA Reading 2 | Average DNA ng/μl | RNA Reading 1 | RNA Reading 2 | Average RNA ng/μl | RIN |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 20180922 | Montipora | 1652 | 395 | soft | 23.4 | 23.4 | 23.4 | 29.6 | 29.6 | 29.6 | 9.1 |
| 20180922 | Montipora | 1652 | 396 | hard | 7.4 | 7.38 | 7.39 | 18.8 | 18.8 | 18.8 | NA |
| 20180922 | Montipora | 1218 | 397 | soft | 19.6 | 19.5 | 19.55 | 30.6 | 30.6 | 30.6 | 9.1 |
| 20180922 | Montipora | 1218 | 398 | hard | 13.4 | 13.4 | 13.4 | 15.2 | 15.4 | 15.3 | NA |
| 20180922 | Montipora | 1037 | 401 | soft | 18.9 | 18.9 | 18.9 | 14.4 | 14.4 | 14.4 | 8.3 |
| 20180922 | Montipora | 1037 | 402 | hard | 9.38 | 9.34 | 9.36 | 11 | 11 | 11 | NA |
| 20180922 | Pocillopora | 1701 | 403 | soft | 107 | 107 | 107 | 109 | 110 | 109.5 | 8.7 |
| 20180922 | Pocillopora | 1701 | 404 | hard | 34.2 | 34.2 | 34.2 | 77 | 77 | 77 | NA |
| 20180922 | Pocillopora | 1581 | 405 | soft | 43.2 | 43.2 | 43.2 | 70.8 | 70.8 | 70.8 | 8.8 |
| 20180922 | Pocillopora | 1581 | 406 | hard | 37.2 | 37.2 | 37.2 | 52.2 | 52.2 | 52.2 | NA |
| 20180922 | Montipora | 1328 | 407 | soft | 24.2 | 22.8 | 23.5 | 27.2 | 27 | 27.1 | 9.1 |
| 20180922 | Montipora | 1328 | 408 | hard | 13.7 | 13.7 | 13.7 | 19.8 | 19.8 | 19.8 | NA |
| 20180923 | Montipora | 1706 | 409 | soft | 12 | 12 | 12 | 14.4 | 14.4 | 14.4 | 8.8 |
| 20180923 | Montipora | 1706 | 410 | hard | 10.7 | 11 | 10.85 | 12.2 | 12.2 | 12.2 | NA |
| 20180923 | Montipora | 2986 | 411 | soft | 17.9 | 17.8 | 17.85 | 10 | 10 | 10 | ** |
| 20180923 | Montipora | 2986 | 412 | hard | 10.3 | 10.2 | 10.25 | 11.6 | 11.8 | 11.7 | NA |
| 20180923 | Pocillopora | 1059 | 413 | soft | 27 | 27 | 27 | 73.2 | 73.2 | 73.2 | 9.2 |
| 20180923 | Pocillopora | 1059 | 414 | hard | 25.2 | 25.2 | 25.2 | 48 | 48 | 48 | NA |
| 20180923 | Pocillopora | 2087 | 415 | soft | 50.6 | 50.4 | 50.5 | 80.6 | 80.6 | 80.6 | 8.9 |
| 20180923 | Pocillopora | 2087 | 416 | hard | 35.4 | 35.2 | 35.3 | 70.8 | 70.8 | 70.8 | NA |
Link to 20190827 TapeStation report, Extractions #395-416
#315 and #313 on the TapeStation are mislabeled. Those samples are #413 and #415.
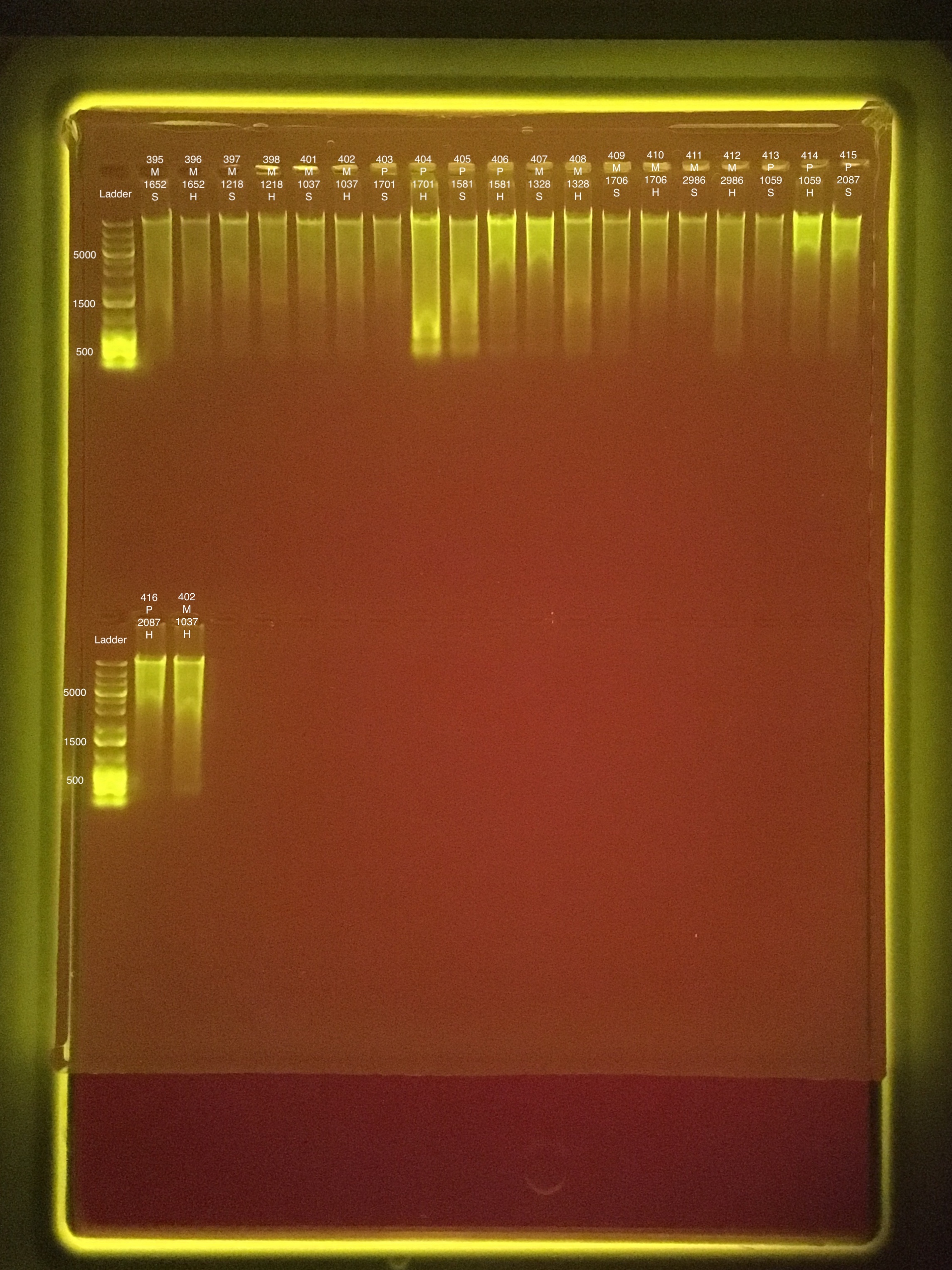
The last lane is a re-do of Extraction #402 because the first lane mostly floated out of the well.